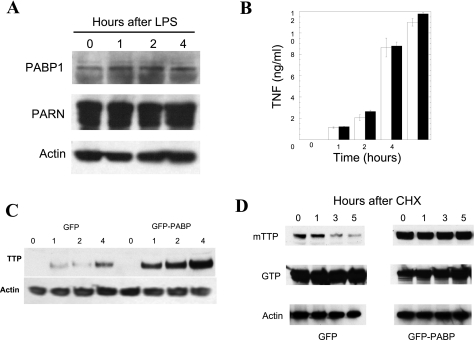
Fig. 5.
PABP1 alters TTP kinetics in cells. PABP1 or PARN protein levels are not appreciably altered by LPS stimulation in RAW264.7 macrophage cells by Western blotting (A). The interaction between TTP and PABP1 was therefore explored in RAW264.7 cells that were stably transfected with either GFP-PABP or GFP. Pools of G418-selected cells were used to avoid site-integration and selection effects with prolonged propagation. E. coli LPS stimulation of these cells was performed from 0 to 6 h and the supernatants were assayed for TNF ELISA (B). A TTP Western blot of LPS-stimulated GFP- and GFP-PABP expressing cells, with equal protein loading verified by actin immunoblotting, is shown in C. To determine whether PABP expression affects TTP protein levels, the kinetics of TTP in the presence of PABP expression was explored in 293T cells cotransfected with myc epitope-tagged TTP (mTTP) and either GFP or GFP-PABP, with protein synthesis subsequently inhibited with cycloheximide (CHX) for 0 to 5 h to inhibit further protein synthesis (D). Loading was confirmed with an anti-actin antibody, TTP expression with the 9E10 monoclonal anti-myc antibody, and the transfection of GFP was verified by use of an anti-GFP antibody (shown at ∼32 kDa for GFP and ∼100 kDa for GFP-PABP). This experiment has been performed >3 times with equivalent results.