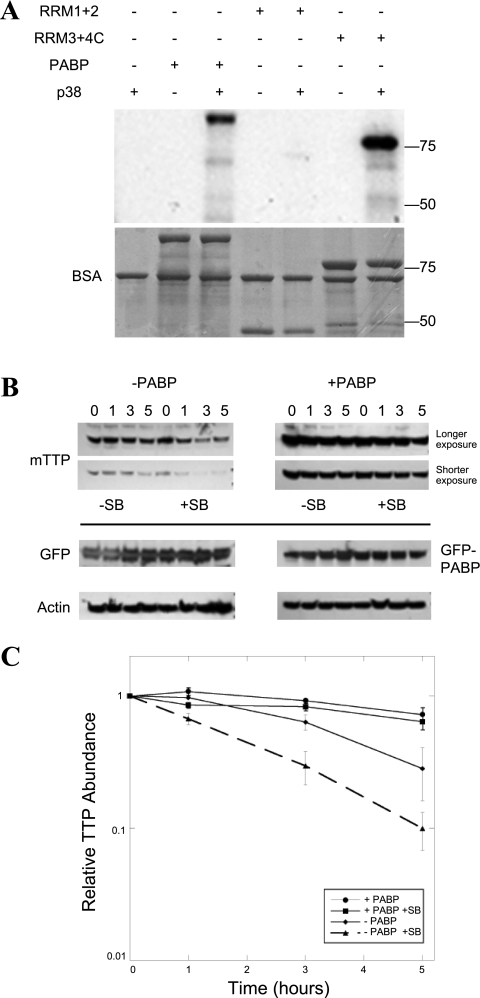
Fig. 6.
Loss of p38 MAP kinase activity destabilizes TTP protein and is inhibited by PABP coexpression, another p38 target. The p38 pathway prevents ARE-directed deadenylation (13), at least in part by promoting rapid TTP degradation by the proteasome (6). We previously mapped p38 phosphorylation sites on TTP (10) and predicted that p38 phosphorylation of PABP might also be possible, given primary sequence of the protein. A: recombinant p38 MAP kinase was used to phosphorylate full-length PABP and the truncated PABP fragments from Fig. 4A in an in vitro kinase assay, then separated on a protein gel that was both autoradiographed on a PhosphoImager to measure phosphorylation (top) and Coomassie stained (bottom). Kinase assays were performed at 30°C in 25-μl reactions containing (final concentrations) 25 mM HEPES, pH 7.4, 10 mM β-glycerophosphate, 2 mM dithiothreitol, 0.1 mg/ml bovine serum albumin, 0.1 mg/ml substrate protein, 10 μg/ml p38 MAP kinase, 10 mM MgCl2, 50 μM [γ-32P]ATP (∼4,000 cpm/pmol). The effect of p38 inhibition on the stabilization of TTP by PABP coexpression was assayed as in Fig. 5E. The kinetics of TTP in the presence of PABP expression was explored in 293T cells cotransfected with myc epitope-tagged TTP (mTTP) and either GFP or GFP-PABP. Protein synthesis was inhibited with CHX for 0–5 h (B), except that parallel wells were also incubated with the p38 MAP kinase inhibitor, SB 203580 (SB). Western blotting was performed as above, with each Western for a given antibody developed on the same chemiluminescent film. Two exposures of mTTP are shown, so that the relative rates of mTTP elimination could be compared between images of comparable intensity at time 0. C: this experiment has been performed >3 times with equivalent results. The densitometry of the TTP films is expressed as means ± SE.