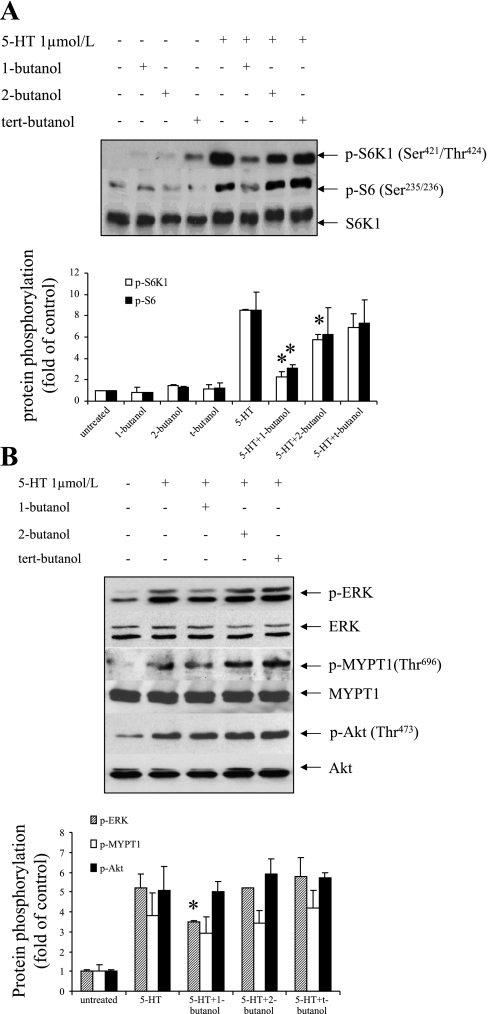
Fig. 3.
Influence of PLD activation on S6K1, mitogen-activated protein kinase (MAPK), Rho kinase (ROCK), and protein kinase B (Akt) pathways. Quiescent SMCs were pretreated with 0.1% 1-butanol, 2-butanol, and tert-butanol for 30 min and then incubated with 1 μmol/l 5-HT for 10 min. Phosphorylation of S6K1 (421Thr/424Ser), S6 ribosomal protein (235Ser/236Ser), extracellular signal-regulated kinase (ERK) (Thr202/Tyr204), MYPT1 (696Thr), and Akt (473Ser) was determined by Western blot analysis using phosphospecific antibodies. The levels of total S6K1 (A) and ERK, MYPT1, and Akt (B) in the whole cell lysates were determined by Western blot analysis using antibodies to these molecules for loading control. All experiments were repeated three times and presented as a representative blot. The band densitometries were calculated as the relative fold in band intensity compared with untreated controls. The bar graphs for blots of A and B represent means ± SD for n = 3. *Significant difference from 5-HT-treated cells (P < 0.05).