Abstract
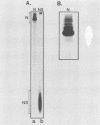
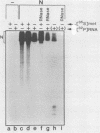
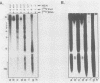
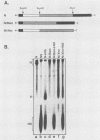
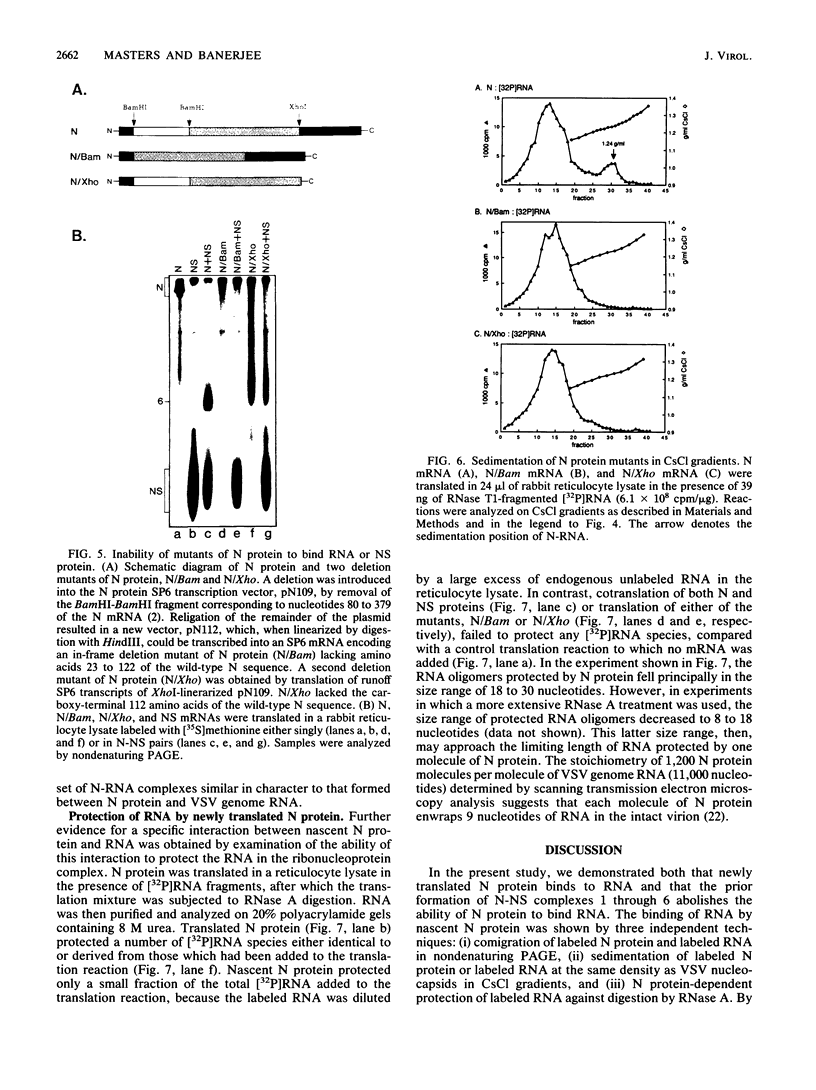
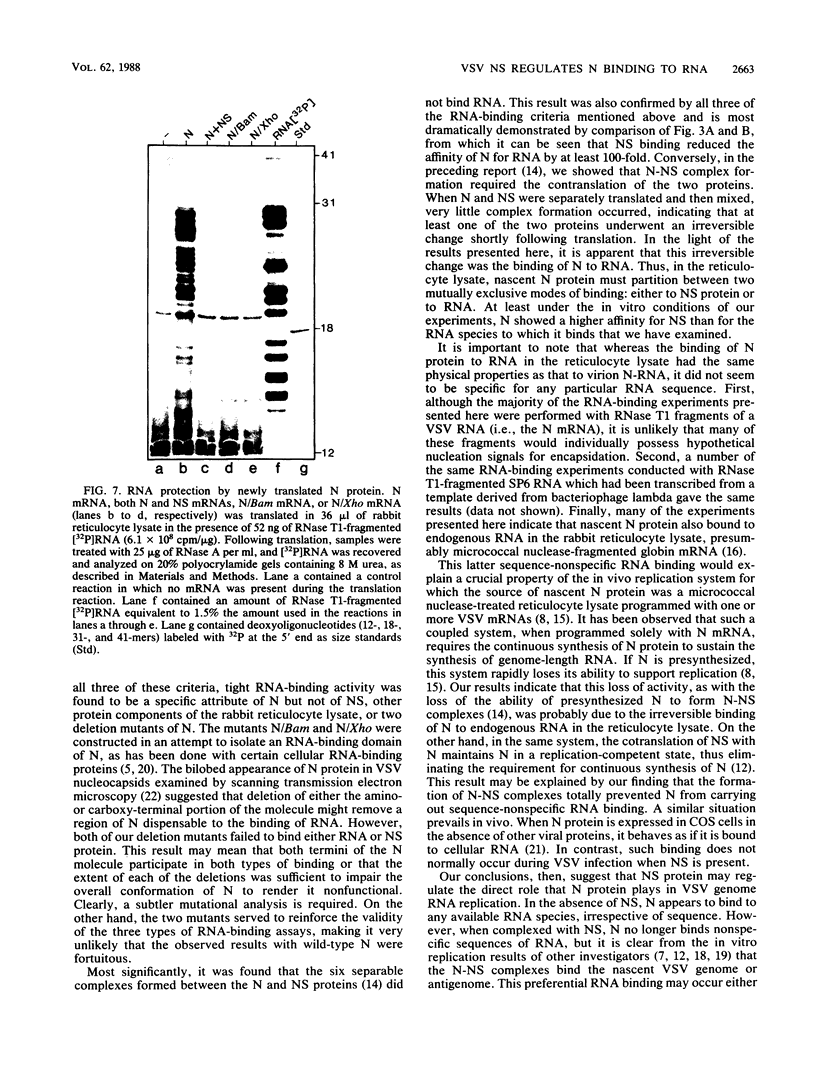
The interactions between the nucleocapsid protein N and either RNA or the phosphoprotein NS of vesicular stomatitis virus (VSV) were studied by the transcription of N and NS mRNAs from SP6 vectors, followed by translation in a rabbit reticulocyte lysate. Nascent N protein bound tightly to added labeled RNA, as well as to endogenous RNA in the reticulocyte lysate. This binding was demonstrated by three independent techniques. First, labeled N protein and labeled RNA migrated identically as a series of sharp, closely spaced bands in a nondenaturing gel system. Second, translated N protein behaved as a stable ribonucleoprotein complex in CsCl gradients and sedimented to the same density as the authentic N-RNA template of VSV. Third, translated N protein protected a series of labeled RNA fragments from digestion by RNase A. None of the three RNA-binding criteria was satisfied by either translated NS protein or two deletion mutants of N protein or by other components of the reticulocyte lysate. The evidence suggests that the observed binding of RNA by nascent N was not RNA sequence specific, in contrast to the encapsidation process during VSV replication. Moreover, the prior formation of N-NS complexes totally abolished the observed binding of RNA by N. Thus, we propose that NS may be responsible for conferring the sequence specificity of the RNA binding that occurs during VSV genome replication.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banerjee A. K. Transcription and replication of rhabdoviruses. Microbiol Rev. 1987 Mar;51(1):66–87. doi: 10.1128/mr.51.1.66-87.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumberg B. M., Giorgi C., Kolakofsky D. N protein of vesicular stomatitis virus selectively encapsidates leader RNA in vitro. Cell. 1983 Feb;32(2):559–567. doi: 10.1016/0092-8674(83)90475-0. [DOI] [PubMed] [Google Scholar]
- Blumberg B. M., Leppert M., Kolakofsky D. Interaction of VSV leader RNA and nucleocapsid protein may control VSV genome replication. Cell. 1981 Mar;23(3):837–845. doi: 10.1016/0092-8674(81)90448-7. [DOI] [PubMed] [Google Scholar]
- Bugler B., Bourbon H., Lapeyre B., Wallace M. O., Chang J. H., Amalric F., Olson M. O. RNA binding fragments from nucleolin contain the ribonucleoprotein consensus sequence. J Biol Chem. 1987 Aug 15;262(23):10922–10925. [PubMed] [Google Scholar]
- Chattopadhyay D., Banerjee A. K. Phosphorylation within a specific domain of the phosphoprotein of vesicular stomatitis virus regulates transcription in vitro. Cell. 1987 May 8;49(3):407–414. doi: 10.1016/0092-8674(87)90293-5. [DOI] [PubMed] [Google Scholar]
- Davis N. L., Arnheiter H., Wertz G. W. Vesicular stomatitis virus N and NS proteins form multiple complexes. J Virol. 1986 Sep;59(3):751–754. doi: 10.1128/jvi.59.3.751-754.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis N. L., Wertz G. W. Synthesis of vesicular stomatitis virus negative-strand RNA in vitro: dependence on viral protein synthesis. J Virol. 1982 Mar;41(3):821–832. doi: 10.1128/jvi.41.3.821-832.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Schubert M. Location of the binding domains for the RNA polymerase L and the ribonucleocapsid template within different halves of the NS phosphoprotein of vesicular stomatitis virus. Proc Natl Acad Sci U S A. 1987 Aug;84(16):5655–5659. doi: 10.1073/pnas.84.16.5655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emerson S. U., Yu Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J Virol. 1975 Jun;15(6):1348–1356. doi: 10.1128/jvi.15.6.1348-1356.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gill D. S., Chattopadhyay D., Banerjee A. K. Identification of a domain within the phosphoprotein of vesicular stomatitis virus that is essential for transcription in vitro. Proc Natl Acad Sci U S A. 1986 Dec;83(23):8873–8877. doi: 10.1073/pnas.83.23.8873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masters P. S., Banerjee A. K. Phosphoprotein NS of vesicular stomatitis virus: phosphorylated states and transcriptional activities of intracellular and virion forms. Virology. 1986 Oct 30;154(2):259–270. doi: 10.1016/0042-6822(86)90452-6. [DOI] [PubMed] [Google Scholar]
- Masters P. S., Banerjee A. K. Resolution of multiple complexes of phosphoprotein NS with nucleocapsid protein N of vesicular stomatitis virus. J Virol. 1988 Aug;62(8):2651–2657. doi: 10.1128/jvi.62.8.2651-2657.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton J. T., Davis N. L., Wertz G. W. N protein alone satisfies the requirement for protein synthesis during RNA replication of vesicular stomatitis virus. J Virol. 1984 Feb;49(2):303–309. doi: 10.1128/jvi.49.2.303-309.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelham H. R., Jackson R. J. An efficient mRNA-dependent translation system from reticulocyte lysates. Eur J Biochem. 1976 Aug 1;67(1):247–256. doi: 10.1111/j.1432-1033.1976.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Peluso R. W., Moyer S. A. Initiation and replication of vesicular stomatitis virus genome RNA in a cell-free system. Proc Natl Acad Sci U S A. 1983 Jun;80(11):3198–3202. doi: 10.1073/pnas.80.11.3198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluso R. W., Moyer S. A. Viral proteins required for the in vitro replication of vesicular stomatitis virus defective interfering particle genome RNA. Virology. 1988 Feb;162(2):369–376. doi: 10.1016/0042-6822(88)90477-1. [DOI] [PubMed] [Google Scholar]
- Sachs A. B., Davis R. W., Kornberg R. D. A single domain of yeast poly(A)-binding protein is necessary and sufficient for RNA binding and cell viability. Mol Cell Biol. 1987 Sep;7(9):3268–3276. doi: 10.1128/mcb.7.9.3268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprague J., Condra J. H., Arnheiter H., Lazzarini R. A. Expression of a recombinant DNA gene coding for the vesicular stomatitis virus nucleocapsid protein. J Virol. 1983 Feb;45(2):773–781. doi: 10.1128/jvi.45.2.773-781.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D., Newcomb W. W., Brown J. C., Wall J. S., Hainfeld J. F., Trus B. L., Steven A. C. Mass and molecular composition of vesicular stomatitis virus: a scanning transmission electron microscopy analysis. J Virol. 1985 May;54(2):598–607. doi: 10.1128/jvi.54.2.598-607.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]