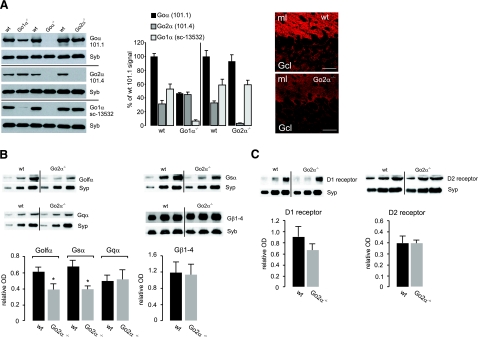
Figure 3.
Expression of trimeric G-protein subunits and D1- and D2-receptor proteins in wild-type (wt) and Go2α−/− mice. A) Ratio of Go1α and Go2α expression. Blots: synaptosomal fractions (P2) from whole brains of Go1α−/− (P20), Goα−/− (P30), or Go2α−/− (adult) and of the corresponding wt littermates were analyzed using the monoclonal antibody clone 101.1 (recognizing both splice variants), the Go2α-specific clone 101.4 (only applicable for Western blot analysis), and the sc13532 Goα antibody preferentially recognizing Go1α. Note that in Goα−/− preparations, none of the antibodies gives a signal, whereas signals for clone 101.4 or sc13532 antibodies are almost or completely absent in Go2α−/− or Go1α−/− mice, respectively. Go2α represents one-third of Goα in P20 (left bars) or adult (right bars) brains, as revealed by quantification using synaptobrevin (Syb) as an internal standard. Go1α is not up-regulated in Go2α−/− mice compared to wt littermates. Immunofluorescence: cerebellar sections were labeled by an antibody (clone 101.1), recognizing both Goα subunits (15). The mean immunofluorescence/100 μm2 of Goα subunits checked was significantly reduced in the molecular layer from 105.2 ± 6.8 in wt to 72.8 ± 9.4 in Go2α−/− mice and in the granular cell layer from 41.7 ± 9.0 (wt) to 21.9 ± 1.8 (knockout) (means ± sd). B) Quantification was performed with synaptosomal preparations from striata of four pairs of wt and Go2α−/− mice. The amounts of Golfα and Gsα, but not of Gqα or Gβ subunits, in general (Gβ1–4), were reduced in Go2α−/− mice. C) There was no significant difference in the amounts of D1- and D2-receptor protein between wt and Go2α−/− mice. For each quantification in B and C, 5, 10, and 15 μg of protein of the respective sample was loaded. Quantification is given as relative optical density (OD) and was performed using synaptophysin (Syp) or synaptobrevin (Syb) as an internal control. Graphs show the quantification from the 10 μg protein load. Values are expressed as means ± sd. *P < 0.05.