Abstract
The authors report on an investigation into recent advances in rabies treatment designed to assess the various claims put forward, their relative merits, and whether they hold true when Indian strains of street virus are used for challenge. The results are presented of treatment with antirabies serum alone, with 5% Semple vaccine, with a combination of serum and vaccination, and with HEP Flury vaccine with and without serum.
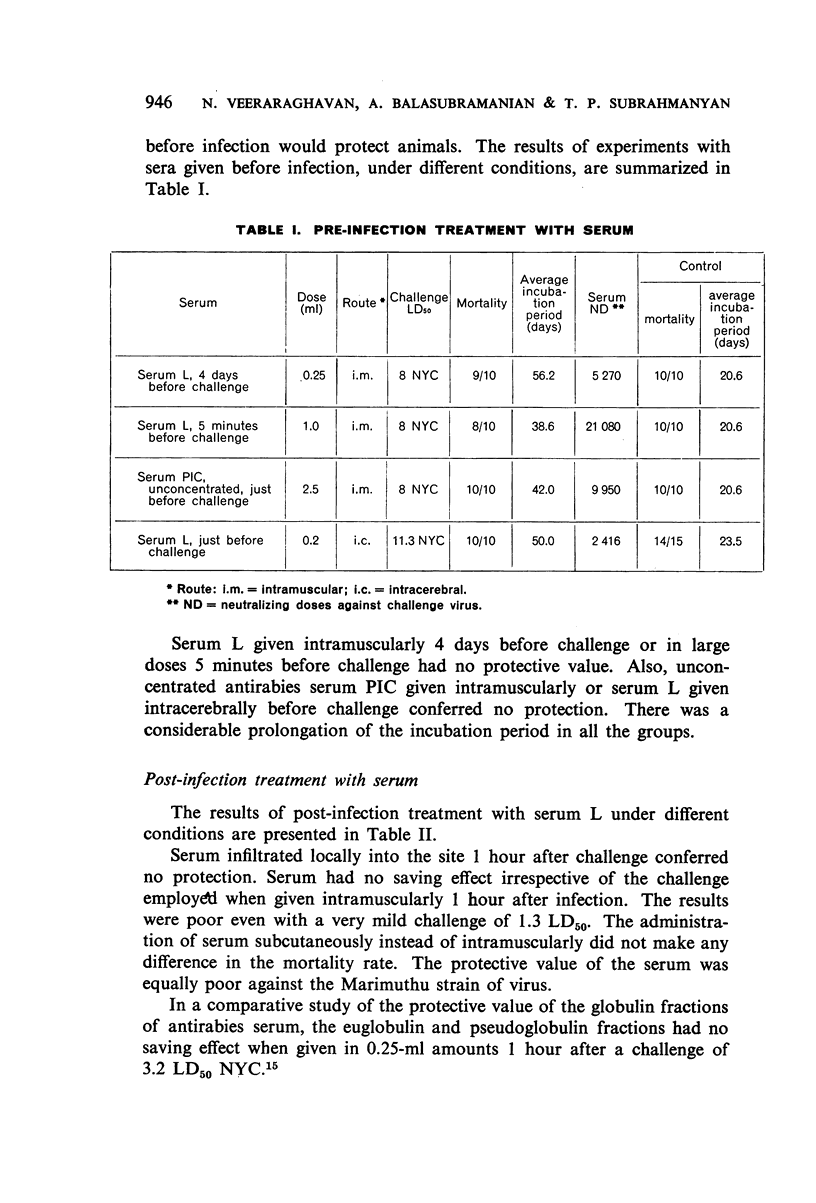
Antirabies serum alone, given before or after infection, while definitely prolonging the incubation period, had no saving effect. Serum seemed to confer some protection when it was obtained from animals of the same species which had survived an infection with the particular challenge virus.
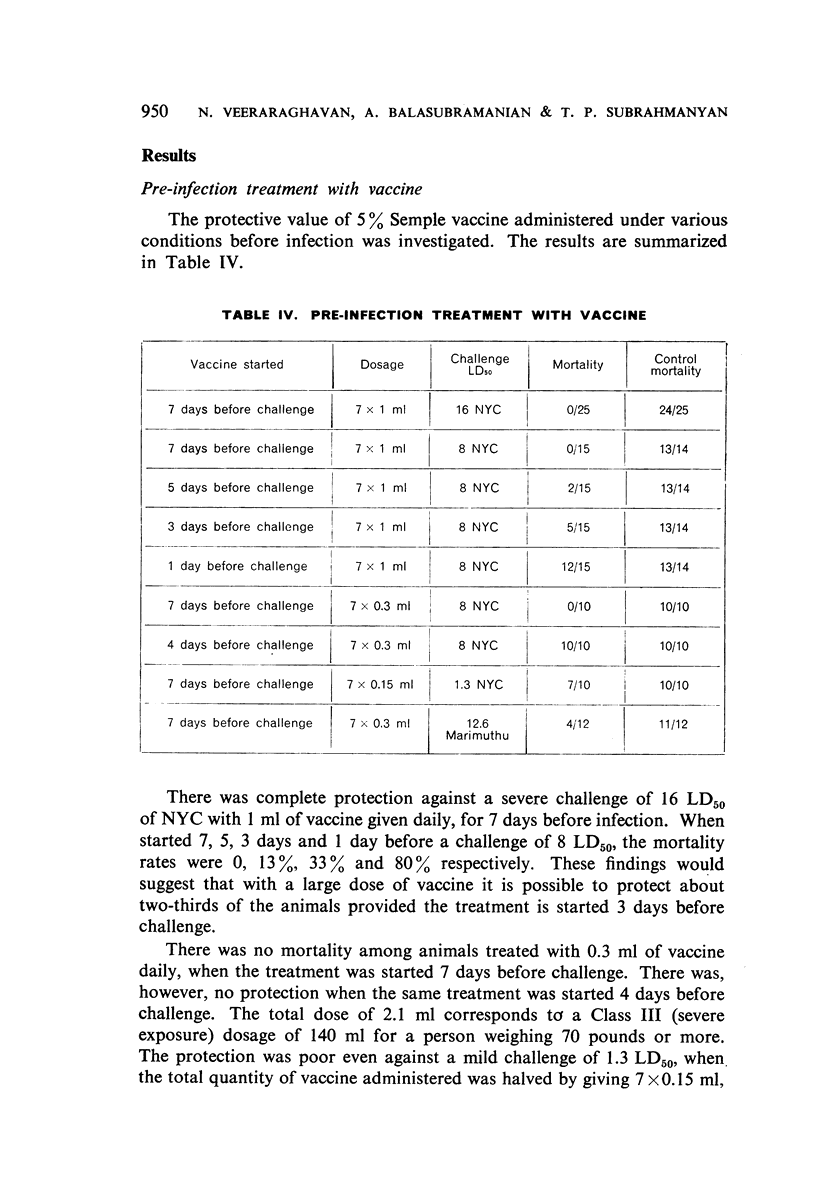
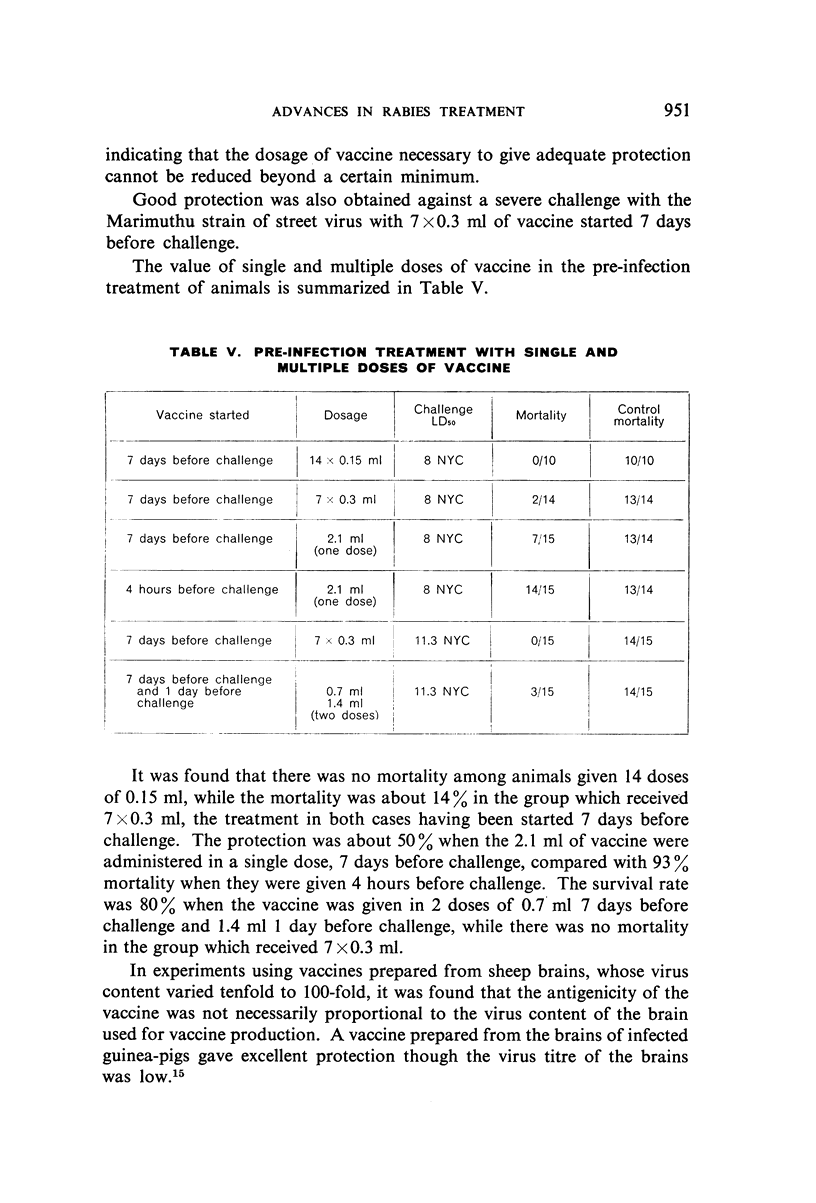
With doses of 5% Semple vaccine comparable to those administered to human beings it was possible to confer solid protection to animals against virulent strains of street virus provided the treatment was started 7 days before challenge.
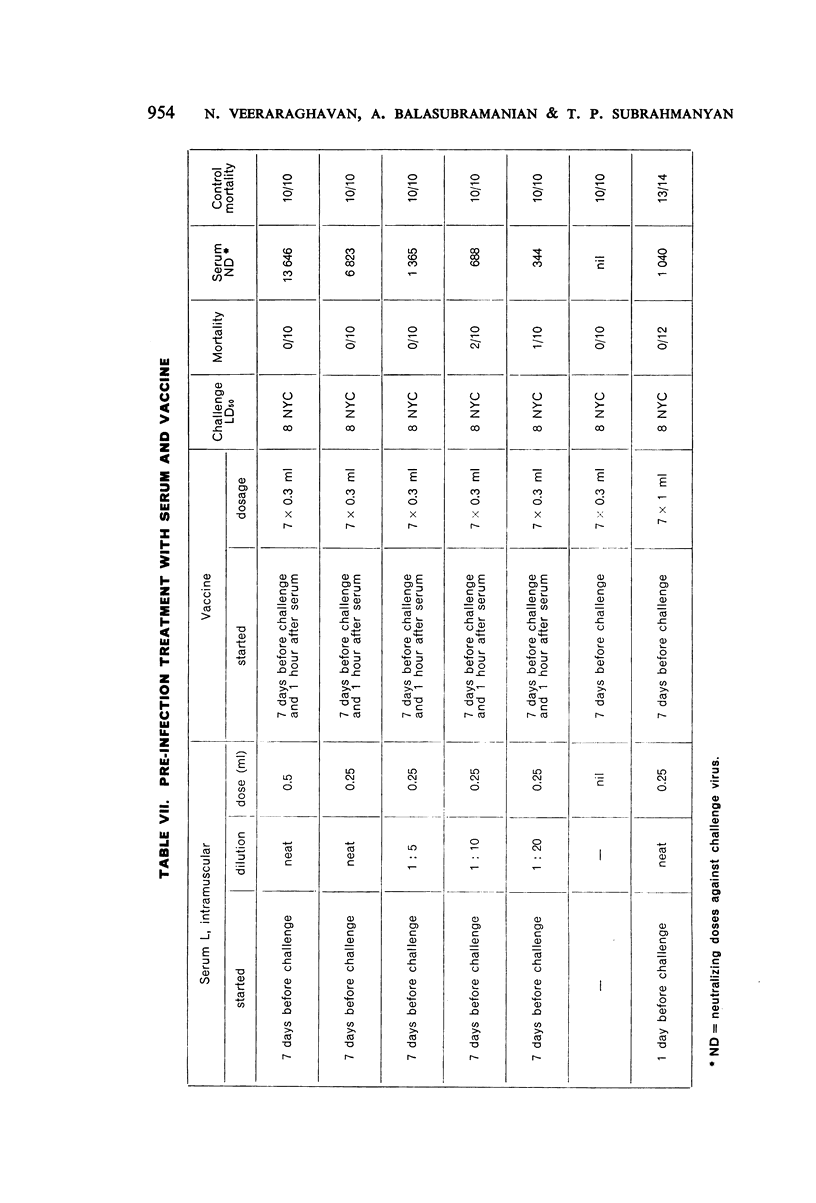
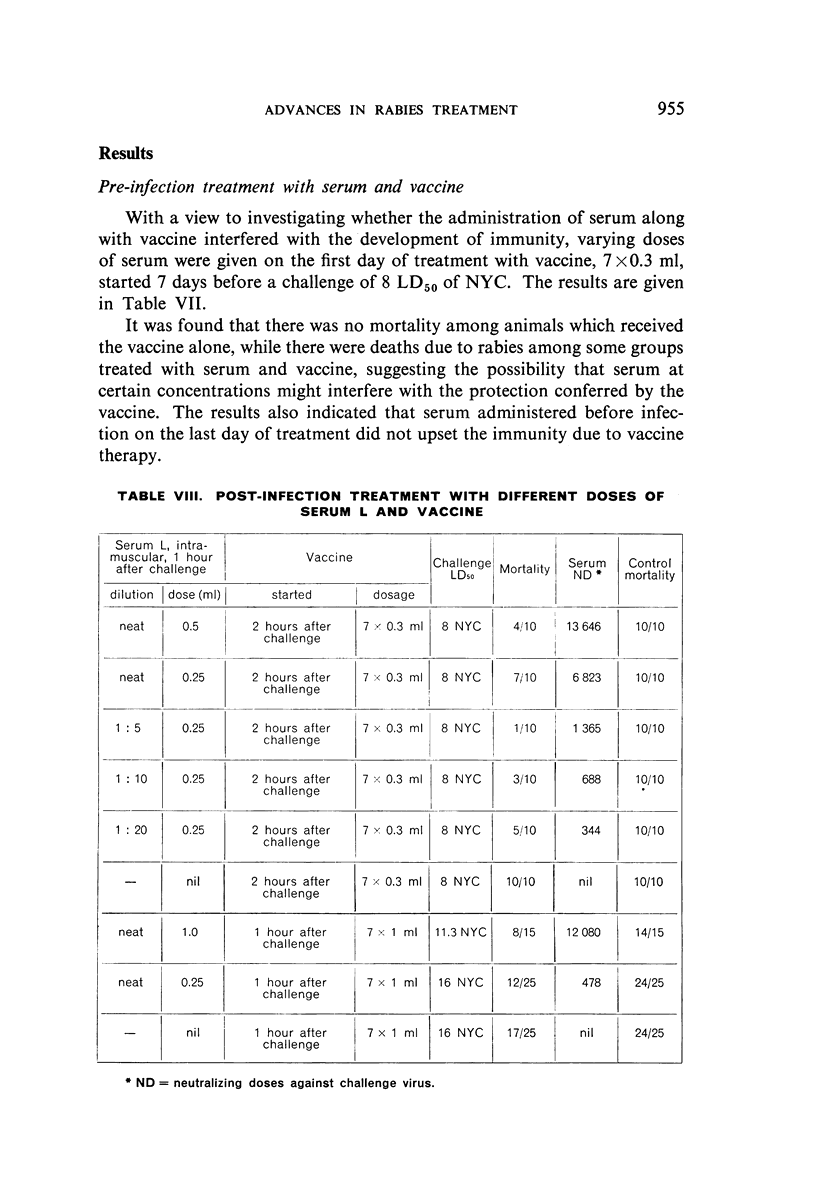
Combined therapy with serum and vaccine given after infection was of great value under certain conditions. There appeared to be an optimum relationship between the quantity of serum given and the antigenicity and dosage of vaccine administered.
HEP Flury vaccine given before infection gave very good protection. But serum and HEP Flury vaccine administered after infection was not of value against an Indian strain of street virus.
Full text
PDF














Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AHUJA M. L., D'SILVA C. B., BROOKS A. G., THOMAS A. K. Studies on rabies. Indian J Med Res. 1951 Jul;39(3):433–444. [PubMed] [Google Scholar]
- HABEL K. Effect on immunity to challenge and antibody response of variation in dosage schedule of rabies vaccine in mice. Bull World Health Organ. 1956;14(4):613–616. [PMC free article] [PubMed] [Google Scholar]
- KOPROWSKI H., BLACK J. Studies on chick-embryo-adapted rabies virus. IV. Immunization of guinea-pigs and description of a potency control test. J Immunol. 1954 Jan;72(1):79–84. [PubMed] [Google Scholar]
- KOPROWSKI H., BLACK J. Studies on chick-embryo-adapted rabies virus. V. Protection of animals with antiserum and living attenuated virus after exposure to street strain of rabies virus. J Immunol. 1954 Jan;72(1):85–93. [PubMed] [Google Scholar]
- KOPROWSKI H., BLACK J. Studies on chick-embryo-adapted rabies virus. VII. Immunological responses of animals to vaccination with high egg passage Flury strain. J Immunol. 1954 Jun;72(6):503–510. [PubMed] [Google Scholar]
- KOPROWSKI H., VAN DER SCHEER J., BLACK J. Use of hyperimmune anti-rabies serum concentrates in experimental rabies. Am J Med. 1950 Apr;8(4):412–420. doi: 10.1016/0002-9343(50)90224-5. [DOI] [PubMed] [Google Scholar]


