FIG. 3.
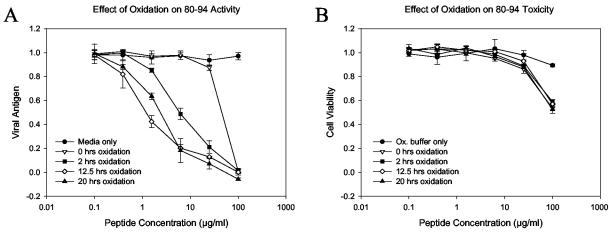

The antiviral potency of 80-94 is dependent on the extent of cysteine oxidation. Peptide 80-94 was purified to >95% purity by reverse-phase HPLC and was then dissolved in oxidation buffer (ammonium bicarbonate buffer [pH 8.0] containing 20% DMSO) to promote disulfide bond formation. At several time points aliquots of peptide solution were removed and assayed for free sulfhydryl content, diluted in PBS, and stored at −20°C. The peptides were later thawed and assayed for antiviral activity. (A) Inhibition curves for peptide aliquots drawn at the indicated time points; (B) cytotoxicity profile of the same aliquots shown in panel A; (C) free sulfhydryl (SH) concentrations of corresponding samples. This pattern of oxidation-dependent enhancement of peptide activity is representative of those from numerous experiments with both crude and highly purified peptides. In panels A and B each datum point represents the mean for three replicate wells, and error bars represent the standard deviations.
