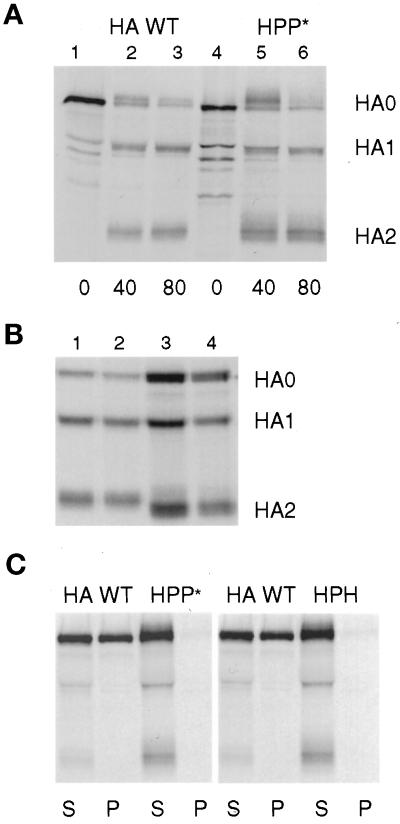
Figure 2.
Characterization of the folding and detergent solubility of chimeric HA proteins. (A) CV-1 cells infected with SV40 vectors expressing HA WT or HPP* were pulse labeled for 15 min and chased for the intervals shown in serum-free DMEM containing 10 μg/ml trypsin. Samples were lysed with both Triton X-100 and SDS and immunoprecipitated. HA0, HA1, and HA2 bands were separated by PAGE under reducing conditions. (B) Cells were pulse labeled for 30 min and chased in DMEM for 60 min and then were treated with trypsin in PBS for 60 min at 4°C. Samples were lysed, precipitated, and analyzed as in A. Lane 1, HA WT; lane 2, HHP*; lane 3, HA G530W; lane 4, HA G520L. (C) Cells were labeled with radioactive amino acids for 15 min and chased for 60 min. The cells were then incubated 10 min at 22°C in PBS containing trypsin to cleave HA at the cell surface into HA1 and HA2 subunits. The cells were lysed with Triton X-100 at 4°C, and sample lysates were separated by centrifugation into supernatant (S) and pellet (P) fractions. The pellets were then solubilized by the addition of SDS, and both S and P fractions were immunoprecipitated with antibodies specific for HA. For A–C, digital images of PhosphorImager scans of the dried gels are shown.