Abstract
A method for titration of poliomyelitis neutralizing antibodies in disposable plastic panels is described. This method is a modification of those reported from the laboratories of Salk and of McLean, in which antibody is measured by protection of monkey-kidney cell suspensions from the metabolic inhibitory influence of the polioviruses.
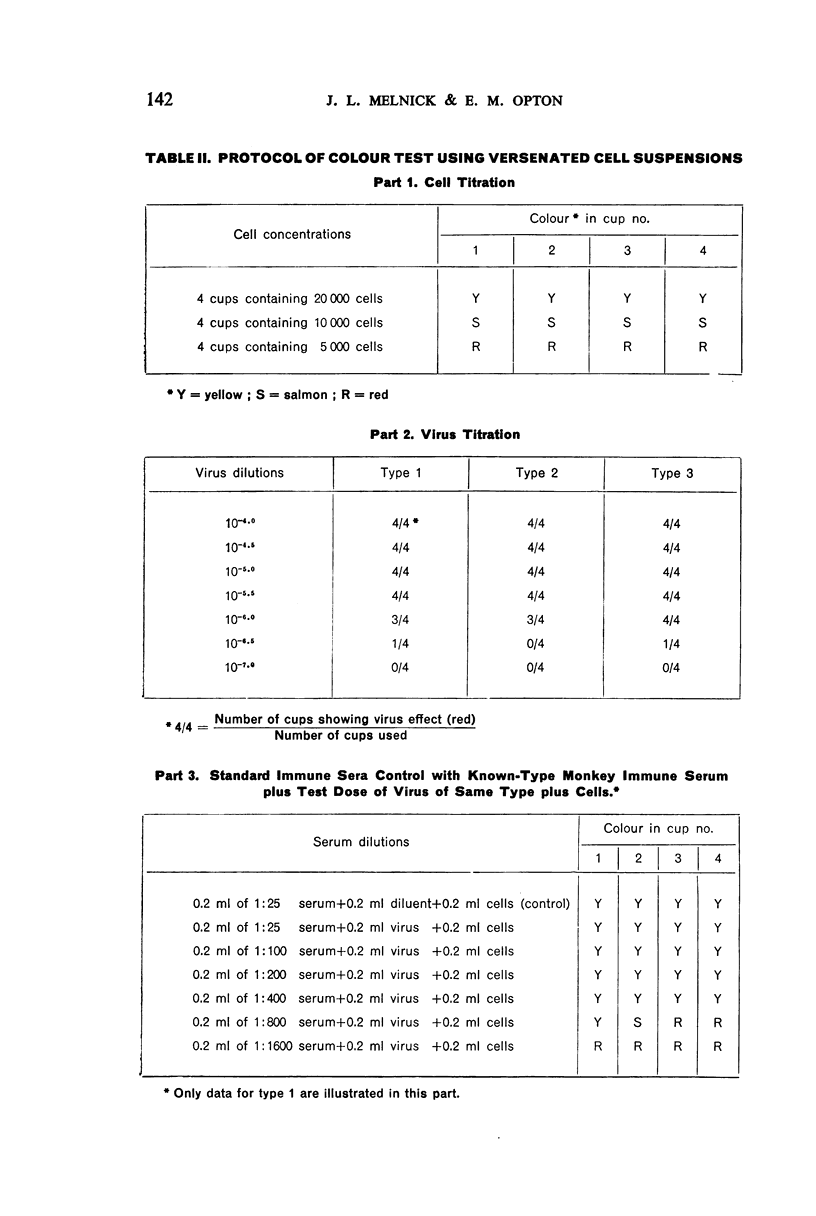
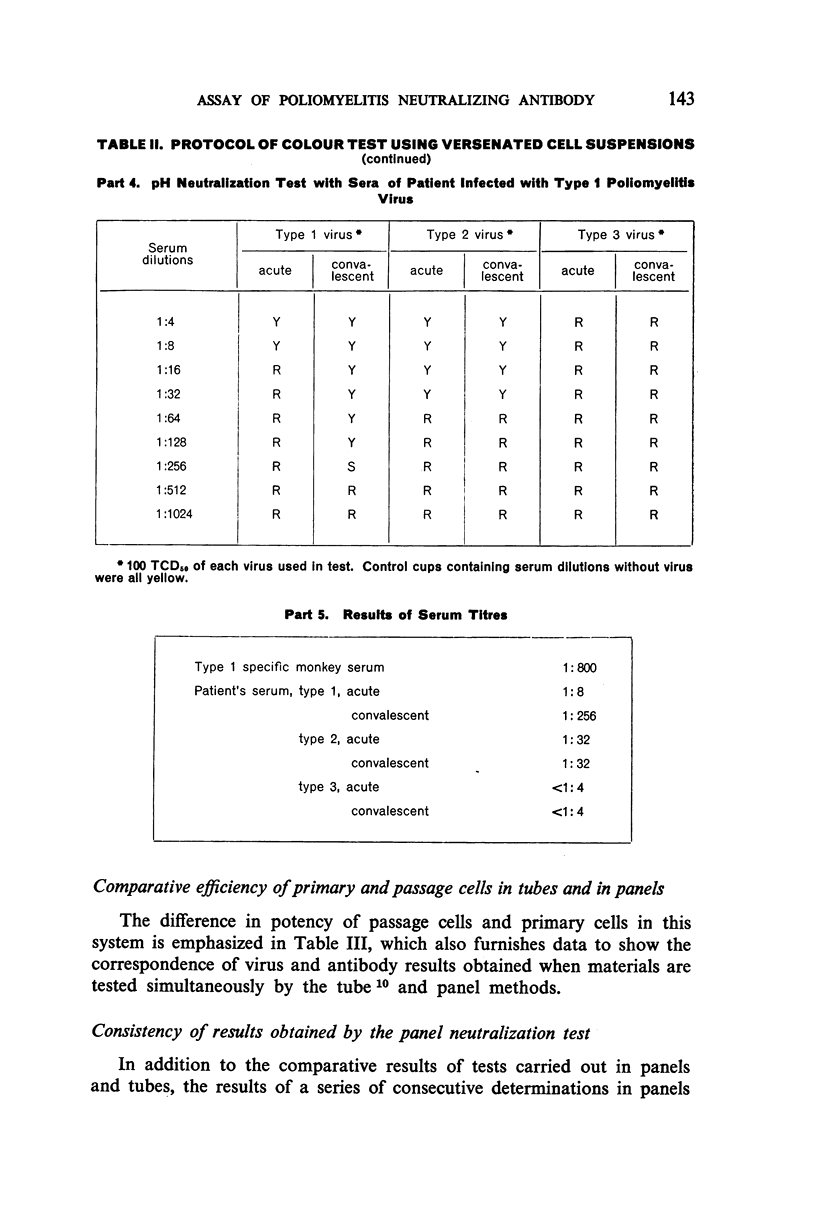
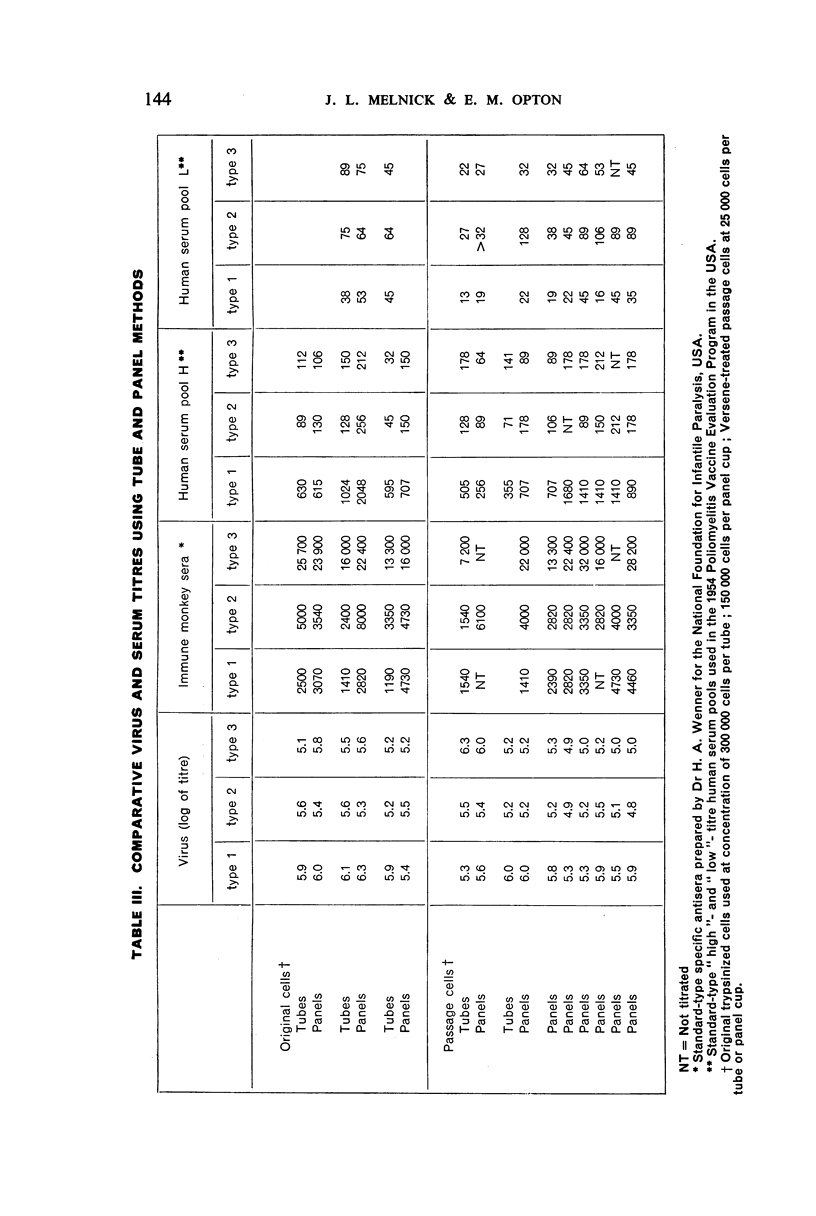
White polystyrene panels, inexpensive and commercially available, are sterilized by alcohol or ultraviolet light. Only 20,000 to 30,000 passage cells are used per cup. The cell suspension is obtained by treatment of primary kidney cultures with a chelating agent, Versene. Such passage cells have a more uniform metabolic activity than trypsinized cells obtained directly from monkey kidney. Cell suspensions may be stored in the refrigerator for up to three weeks and still prove useful for the colour test. They are standardized for each test by a prior titration of their viability, the results of which are available in less than 24 hours. Both for the primary outgrowth from trypsinized monkey kidney and for the colour test itself, the simple lactalbumin hydrolysate medium is used. Panels are sealed with a paraffin oil of high viscosity.
Full text
PDF



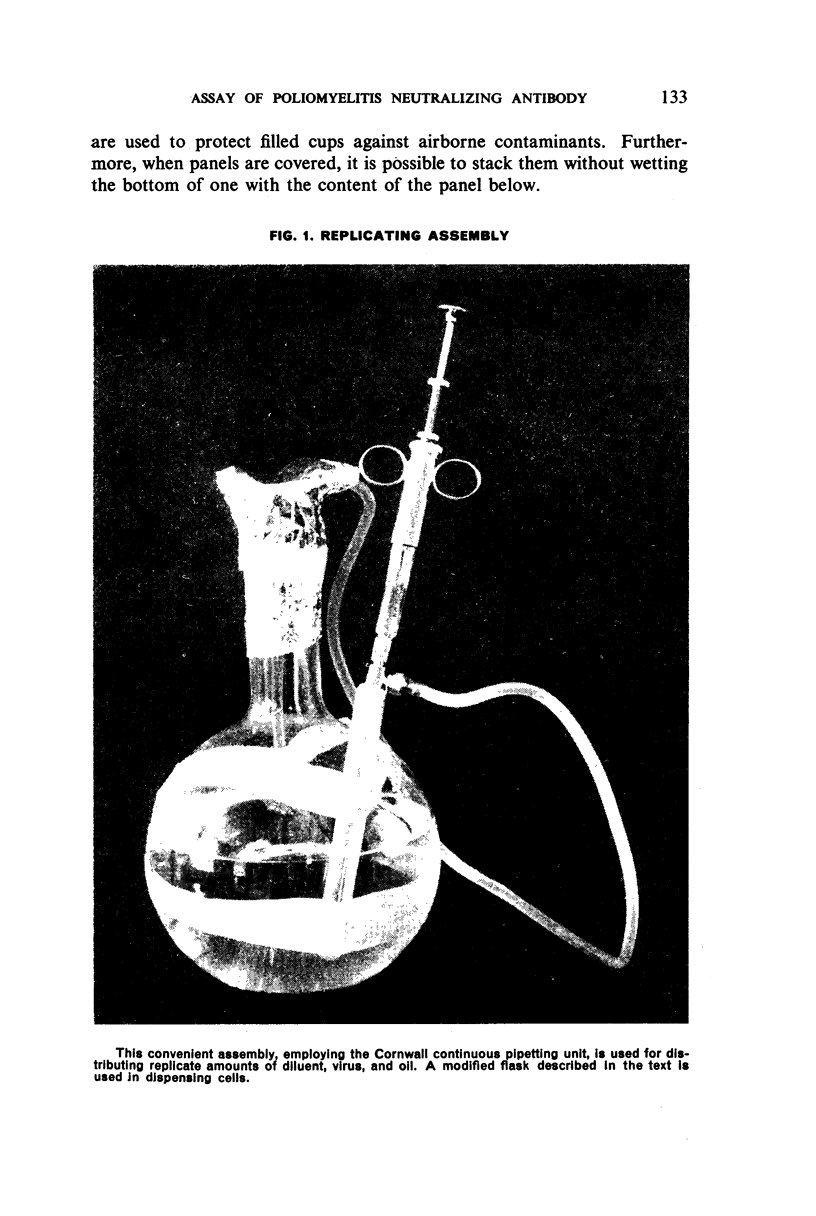
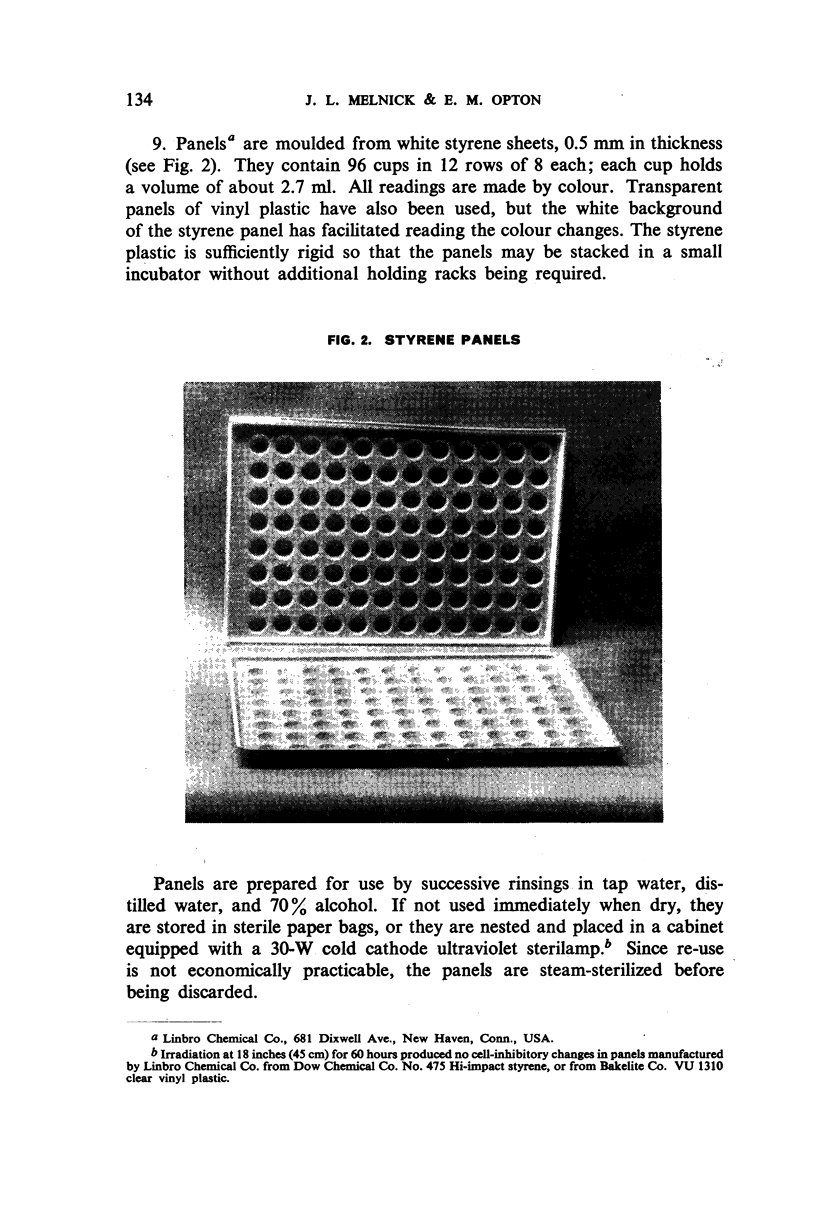




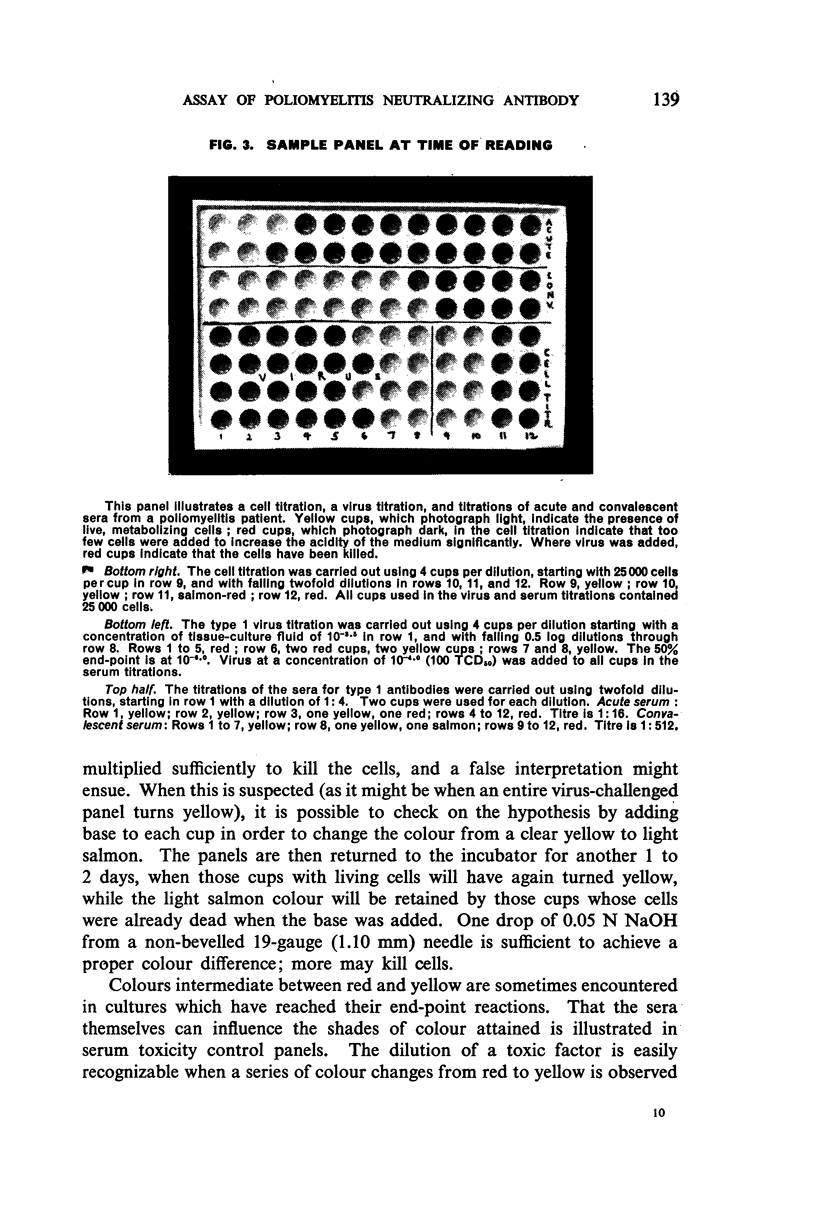
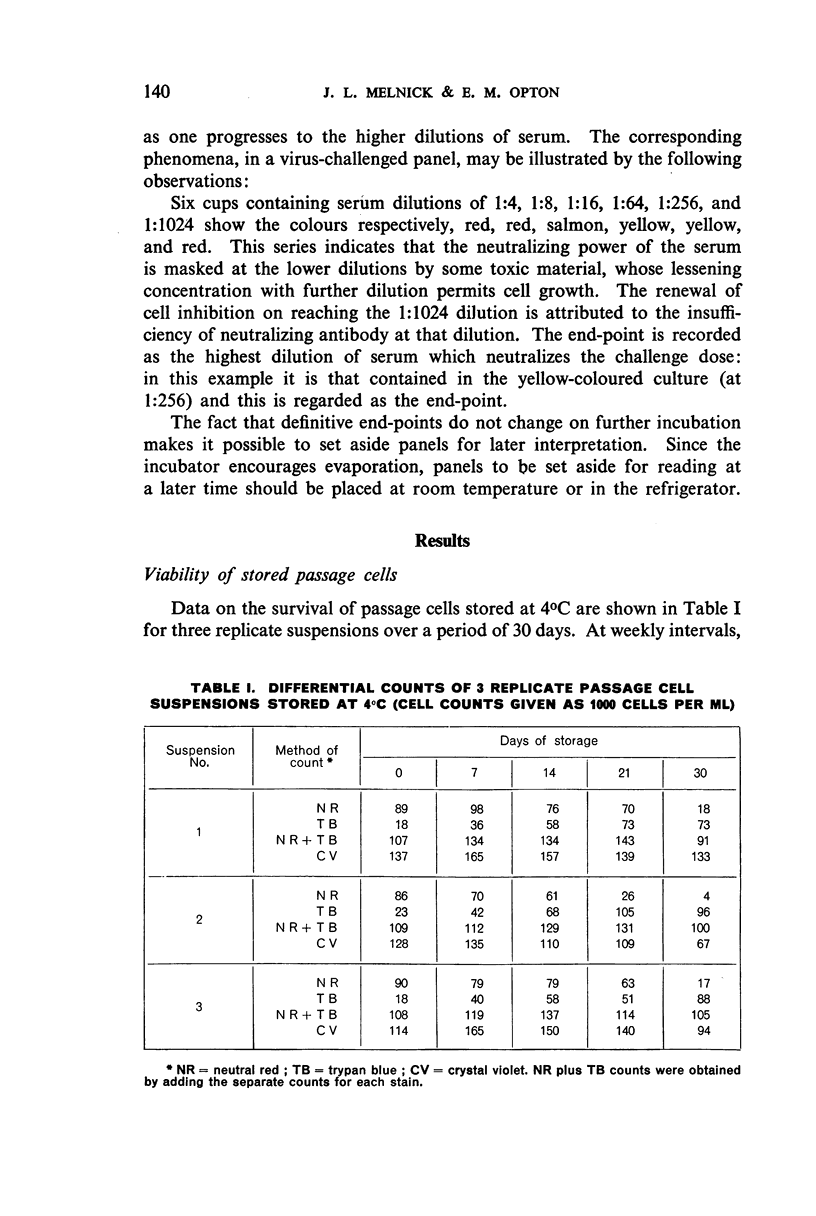



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARSKI G., LEPINE P. Microméthode de séroneutralisation de la poliomyélite; emploi de cultures cellulaires sur plaques moulées de matière plastique. Ann Inst Pasteur (Paris) 1954 Jun;86(6):693–701. [PubMed] [Google Scholar]
- ENDERS J. F. The application of tissue cultures to the preparation and assay of viral antibodies. Ann N Y Acad Sci. 1954 Nov 17;58(7):1072–1084. doi: 10.1111/j.1749-6632.1954.tb45893.x. [DOI] [PubMed] [Google Scholar]
- LEDINKO N., MELNICK J. L. Poliomyelitis viruses in tissue culture. V. Reaction of virus and antibody; variables of the quantitative neutralization test. Am J Hyg. 1953 Sep;58(2):223–247. [PubMed] [Google Scholar]
- LIPTON M. M., STEIGMAN A. J. A simplified colorimetric test for poliomyelitis virus and antibody. Proc Soc Exp Biol Med. 1955 Jan;88(1):114–118. doi: 10.3181/00379727-88-21508. [DOI] [PubMed] [Google Scholar]
- MELNICK J. L. Tissue culture techniques and their application to original isolation, growth, and assay of poliomyelitis and orphan viruses. Ann N Y Acad Sci. 1955 Sep 27;61(4):754-72; discussion, 772-3. doi: 10.1111/j.1749-6632.1955.tb42532.x. [DOI] [PubMed] [Google Scholar]
- ROBBINS F. C., ENDERS J. F., WELLER T. H. Cytopathogenic effect of poliomyelitis viruses in vitro on, human embryonic tissues. Proc Soc Exp Biol Med. 1950 Nov;75(2):370–374. doi: 10.3181/00379727-75-18202. [DOI] [PubMed] [Google Scholar]
- ROBERTSON H. E., BRUNNER K. T., SYVERTON J. T. Propagation in vitro of poliomyelitis viruses. VII. pH change of HeLa cell cultures for assay. Proc Soc Exp Biol Med. 1955 Jan;88(1):119–122. doi: 10.3181/00379727-88-21509. [DOI] [PubMed] [Google Scholar]
- SALK J. E., YOUNGNER J. S., WARD E. N. Use of color change of phenol red as the indicator in titrating poliomyelitis virus or its antibody in a tissue-culture system. Am J Hyg. 1954 Sep;60(2):214–230. doi: 10.1093/oxfordjournals.aje.a119714. [DOI] [PubMed] [Google Scholar]





