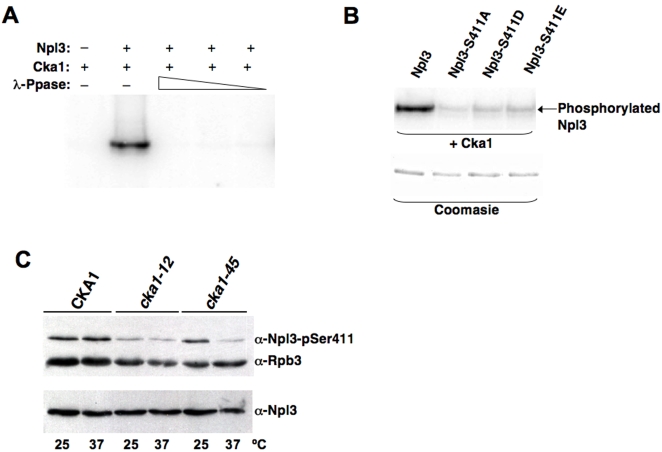
Figure 3. Cka1, the alpha catalytic subunit of CK2, phosphorylates Npl3.
(A) Recombinant His-Cka1 was incubated with His-Npl3 in the presence of radiolabeled ATP. Phosphorylation of Npl3 is reversed by the addition of increasing concentrations of λ-Phosphatase. (B) Using the in vitro kinase assay with mutants of Npl3-S411, this residue was uncovered as an additional phosphorylation site. Wild-type His-Npl3 or S411 point mutants, His-Npl3-S411A, -S411D or -S411E were incubated with His-Cka1, as described for (A) in the presence of radiolabeled-ATP. Coomasie stain representing the concentration of recombinant Npl3 proteins used is shown below. (C) CKA1 mutations show reduced phosphorylation of Npl3. Whole-cell extracts were prepared for cells grown for one hour at the permissive (25°C) or non-permissive (37°C) temperature for wild-type CKA1, cka1-12 or cka1-45, and immunoblot analysis was performed using antibodies specific for phosphorylated or non-phosphorylated Npl3, as indicated. Detection of Rpb3 with specific antibodies is shown as a loading control.