Abstract
We demonstrated recently that P8A-CCL2, a monomeric variant of the chemokine CCL2/MCP-1, is unable to induce cellular recruitment in vivo, despite full activity in vitro. Here, we show that this variant is able to inhibit CCL2 and thioglycollate-mediated recruitment of leukocytes into the peritoneal cavity and recruitment of cells into lungs of OVA-sensitized mice. This anti-inflammatory activity translated into a reduction of clinical score in the more complex inflammatory model of murine experimental autoimmune encephalomyelitis. Several hypotheses for the mechanism of action of P8A-CCL2 were tested. Plasma exposure following s.c. injection is similar for P8A-CCL2 and wild-type (WT) CCL2, ruling out the hypothesis that P8A-CCL2 disrupts the chemokine gradient through systemic exposure. P8A-CCL2 and WT induce CCR2 internalization in vitro and in vivo; CCR2 then recycles to the cell surface, but the cells remain refractory to chemotaxis in vitro for several hours. Although the response to P8A-CCL2 is similar to WT, this finding is novel and suggests that despite the presence of the receptor on the cell surface, coupling to the signaling machinery is retarded. In contrast to CCL2, P8A-CCL2 does not oligomerize on glycosaminoglycans (GAGs). However, it retains the ability to bind GAGs and displaces endogenous JE (murine MCP-1) from endothelial surfaces. Intravital microscopy studies indicate that P8A-CCL2 prevents leukocyte adhesion, while CCL2 has no effect, and this phenomenon may be related to the mechanism. These results suggest that oligomerization-deficient chemokines can exhibit anti-inflammatory properties in vivo and may represent new therapeutic modalities.
Keywords: inflammation, antagonist, obligate monomer, EAE
INTRODUCTION
CCL2/MCP-1 is a member of the CC class of chemokines, a family of small proteins that governs directional cell trafficking through interaction with G protein-coupled receptors and cell-surface proteoglycans [1, 2]. CCL2 mediates the recruitment of several leukocyte populations including monocytes, T lymphocytes, and NK cells [3]. It binds exclusively to the receptor, CCR2, in contrast to many other inflammatory chemokines [4], but CCR2 is also activated by several other chemokines, including CCL8/MCP-2, CCL7/MCP-3, and CCL13/MCP-4 in humans and MCP-2–5 in the murine system [5].
Despite the apparent promiscuity of CCR2, studies using CCR2 and CCL2 knockout mice confirmed the importance of this chemokine:receptor pair in mouse models of inflammatory disease [6,7,8], implicating them in a number of disorders including multiple sclerosis and atherosclerosis. Their role has been validated further in many models of inflammation in rodent and nonhuman primate models, using a variety of different inhibitors. Administration of neutralizing mAb against CCL2 resulted in reduced clinical severity in crescentic glomerulonephritis in mice [9], and an anti-CCR2 antibody reduced neointimal hyperplasia in a nonhuman primate model of in-stent restinosis [10]. Receptor antagonists of CCL2 with truncated amino termini bind CCR2 with high affinity but do not cause signaling and have been used successfully in several disease models. For example, a form of CCL2 missing residues 1–8 significantly reduced the clinical score in the spontaneous onset of arthritis in lpr mice (a mouse strain predisposed to lupus-like syndrome) [11]. Similarly, gene transfer of a truncated variant, 7ND, was shown to be efficacious in a mouse model of atherosclerosis [12, 13]. Interestingly, the small molecule, bindarit, which specifically decreases levels of CCL2 expression [14], was efficacious in vivo in models of acute pancreatitis [15], lupus [16], and adjuvant-induced arthritis [17], despite its relatively low potency in vitro. However, although blockade of CCR2 with a mAb in the model of collagen-induced arthritis prevented disease when administered prophylactically, it aggravated joint inflammation when given in a therapeutic regimen [18]. This was attributed to the expression of CCR2 on regulatory T cells (Tregs) in the mouse and suggests that care should be taken not to interfere with Treg trafficking in autoimmune disease. Unfortunately, the first clinical trial inhibiting the CCL2-CCR2 axis conducted on rheumatoid arthritis patients with an anti-CCL2 antibody was disappointing in that although reduction of macrophage accumulation into the joints was observed, the end-point of reduction in clinical score was not achieved [19].
In addition to receptor binding, glycosaminoglycan (GAG) binding and oligomerization of chemokines have been shown to be important for in vivo biological activity [20]. In the case of CCL5/RANTES, we demonstrated that interference with these properties presents a novel, anti-inflammatory strategy; administration of 44ANAA47-CCL5, a mutant that is crippled in its ability to bind GAGs and to form oligomeric structures, resulted in significantly reduced clinical severity in a murine model of multiple sclerosis, experimental autoimmune encephalomyelitis (EAE) [21]. Here, we investigated the potential therapeutic effect of P8A-CCL2, a point mutant that does not oligomerize but induces cell recruitment as effectively as wild-type (WT) CCL2 in vitro [22] yet is unable to recruit cells into the peritoneal cavity [20]. We show that it is able to inhibit recruitment in several animal models, underscoring the importance of oligomerization. We also investigated potential mechanisms for its inhibitory behavior. The results suggest that a combination of mechanisms may be operative. As P8A-CCL2 binds GAGs and can displace endogenous CCL2/JE from the endothelial surface, we propose that it exhibits anti-inflammatory properties by GAG binding-site competition. Furthermore, as it is signaling-competent, induction of receptor down-regulation could also contribute to its inhibitory properties. Finally, unlike WT CCL2, P8A-CCL2 inhibits leukocyte adhesion through mechanisms that remain to be elucidated.
MATERIALS AND METHODS
Cellular recruitment in vivo
An i.p. recruitment assay was carried out as described previously [20] using 8- to 12-week-old, female Balb/c mice (Janvier, Le Geneset St Isle, France). Administrations were introduced i.p. in a total volume of 200 μl. Control animals were injected with NaCl (0.9%, LPS-free), and recruitment was induced with 0.5 mg/kg CCL2 in NaCl. To test inhibition, P8A-CCL2 was administered i.p. 30 min prior to CCL2 administration at doses ranging from 0.5 to 0.005 mg/kg. At 18 h postinjection, the mice were sacrificed, the peritoneal cavity was washed, and total cells were collected and counted in a Neubauer hemocytometer (Hausser Scientific Co., Horsham, PA, USA). In a second model, thioglycollate (1.5%, 40 ml/kg) was given i.p., and P8A-CCL2 (1–10 mg/kg) or dexamethasone (1 mg/kg) as a positive control compound was administered s.c. 15 min prior to and then at 24 h after thioglycollate administration. At 48 h post-thioglycollate injection, the mice were sacrificed, and the peritoneal cavity was washed using 2 × 5 ml PBS containing 1 mM EDTA, precooled to 4°C. After centrifugation for 10 min at 3000 rpm, the pellet was resuspended in 1 ml PBS, and the cells were counted using a Beckman Coulter counter. In an experiment to determine the pharmacodynamic properties of P8A-CCL2 (see Fig. 3D), a single administration of 10 mg/kg was given at various time-points before the thioglycollate stimulus, and the cells were enumerated at 48 h. In a third model, recruitment into the lungs after OVA sensitization was conducted as described previously [21], and inhibition was monitored following i.p. administration of 0.5 mg/kg P8A-CCL2 compared with 0.5 mg/kg [44AANA47]-CCL5.
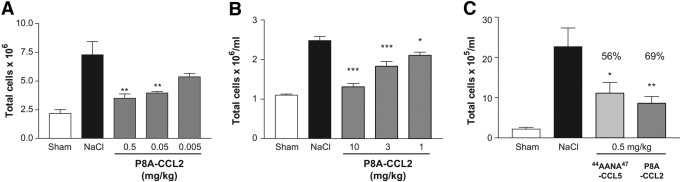
Fig. 1.
P8A-CCL2 inhibits cell recruitment in vivo. Data are expressed as mean total cell counts ± sd, and n = 5 mice per group. White bars indicate cellularity in unsensitized animals, maximal response with NaCl treatment is shown in black, and treatment with various doses of P8A-CCL2 is shown as gray bars. (A) Chemokine-induced peritoneal recruitment model: Cellular recruitment was induced by 10 μg CCL2 i.p., and the cells were enumerated following peritoneal lavage after 18 h as described in the text. P8A-CCL2 was administered i.p. 30 min prior to the CCL2 stimulus at the doses indicated. (B) Thioglycollate-induced recruitment model: Cellular recruitment was induced by thioglycollate into the peritoneal cavity as described in text. P8A-CCL2 was administered s.c. 15 min prior to the thioglycollate stimulus, and a second administration was given after 24 h. Cells were enumerated after 48 h. (C) OVA-sensitization model: Comparison of the inhibitory effect on cellular recruitment into the bronchiolar lavage (BAL) fluid by monomeric P8A-CCL2 (dark-gray bar) and [44AANA47]-CCL5 (light-gray bar) dosed at 0.5 mg/kg i.p. 30 min before challenge in OVA-induced airway inflammation. Inhibition is expressed as percent inhibition compared with the BAL cellularity of the NaCl-treated group as follows: *, P < 0.05; **, P < 0.01; and ***, P < 0.001.
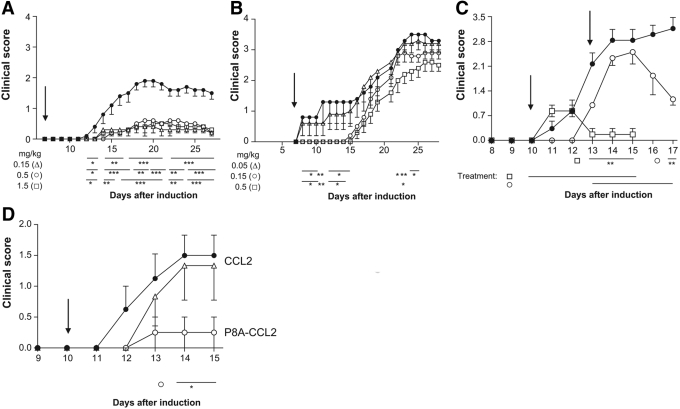
Fig. 2.
Treatment with P8A-CCL2 in the MOG-induced murine model of EAE reduces clinical score. (A) P8A-CCL2 was administered i.p. at Day 7 using the protocol with two MOG sensitizations as described in the text. (•) NaCl-treated control; (▵) 0.15 mg/kg; (○) 0.5 mg/kg; (□) 1.5 mg/kg. (B) P8A-CCL2 was administered i.p. at Day 7 (indicated by arrow) using the protocol with two MOG sensitizations as described in the text. (•) NaCl-treated control; (▵) 0.05 mg/kg; (○) 0.15 mg/kg; (□) 0.5 mg/kg; (C) (•) NaCl-treated control; P8A-CCL2 (1.5 mg/kg) was administered s.c. at Day 10 (□) and at Day 13 (○; indicated by arrow) using the protocol with a single MOG sensitization as described in the text. (D) (•) NaCl-treated control; (○) 1.5 mg /kg P8A-CCL2, and (▵) CCL2 were administered s.c. daily starting at Day 10 (indicated by arrow) using the protocol with a single MOG sensitization as described in the text. Significance is indicated by *, **, and *** for P < 0.05, P < 0.01, and P < 0.001, respectively, over the indicated period of days shown by the bars when compared with vehicle-treated animals.
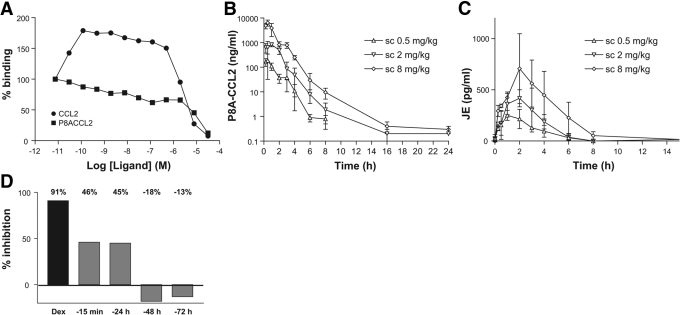
Fig. 3.
P8A-CCL2 displaces murine JE from the endothelial surface. (A) The ability of CCL2 (•) and P8A-CCL2 (▪) to displace (in the case of P8A-CCL2) or increase (in the case of CCL2) the amount of [125I]-CCL2 onto heparin sepharose beads was determined in a competition equilibrium-binding assay. One of two experiments is shown. (B) P8A-CCL2 was administered s.c. at the doses indicated, and its concentration in the serum was determined by ELISA as described in the text at 0.5, 1, 2, 3, 4, 6, 8, 16, and 24 h. (C) The concentration of JE was determined by ELISA at the time-points indicated. (D) In a thioglycollate peritonitis model, a single dose of P8A-CCL2 (10 mg/kg) was given at the time-points indicated prior to the thioglycollate stimulus (gray bars). Dexamethasone (Dex; 1 mg/kg) was used as a positive control for inhibition of cell recruitment and administered s.c. 15 min prior to the thioglycollate stimulus (black bar). Cells were enumerated in the peritoneal cavity 48 h after the thioglycollate administration. The experiment was performed twice.
EAE
Two experimental protocols were used to induce EAE in C57BL/6 mice. In the first protocol [23], EAE was induced with a s.c. flank injection of 200 μg myelin oligodendrocyte glycoprotein (MOG)35–55 peptide in CFA containing 5 mg/ml Mycobacterium tuberculosis H37 RA (Difco Laboratories, Detroit, MI, USA) at Days 0 and 7. Additionally, an i.p. injection of pertussis toxin (PTX; 500 ng in 400 μl) was performed at Days 0 and 2. In the second protocol [24], EAE was induced by s.c. immunization (base of tail) with an emulsion containing 100 μg MOG35–55 peptide, and CFA was supplemented with 4 mg/ml M. tuberculosis. PTX (300 ng/animal) was injected i.p. on the day of immunization and again 48 h later. Mice were treated with P8A-CCL2 at the doses indicated (see Fig. 2). Clinical score was monitored daily, and mice were scored as follows: 0, no sign of disease; 0.5, partial tail paralysis; 1, tail paralysis; 2, partial hind-limb paralysis; 3, complete hind-limb paralysis; 4, hind-limb and fore-limb paralysis; 5, moribund or dead.
Intravital microscopy in mouse brain
Intravital microscopy of the mouse cerebro-microvasculature was performed as described previously [24]. Briefly, EAE was induced as described in the second protocol above. At Day 14 after EAE induction, the pial vasculature was exposed by a craniotomy, and leukocyte/endothelium interactions were observed. Leukocytes were fluorescently labeled by i.v. administration of rhodamine 6G (0.5 mg/kg body weight) and observed using a microscope (Olympus B201, ×20 objective lens, corresponding to 100 μm area) outfitted with a fluorescent light source (epi-illumination at 510–560 nm, using a 590-nm emission filter). Rolling leukocytes were defined as white cells moving at a velocity less than that of erythrocytes. Leukocytes were considered adherent to the venular endothelium if they remained stationary for 30 s or longer. A single administration of P8A-CCL2 or CCL2 (0.5 or 1.5 mg/kg in 200 μl saline) was given s.c. into the nape of the neck 45 min prior to the start of measurements.
Oligomerization on heparin
Oligomerization of P8A-CCL2 and CCL2 on solid-phase heparin was performed as described previously [25]. Briefly, heparin sepharose beads (GE Healthcare Life Sciences, Piscataway, NJ, USA) were incubated with 0.1 nM 125I-CCL2 in binding buffer (50 mM HEPES, pH 7.4, containing 0.5% BSA, 5 mM MgCl2, and 1 mM CaCl2) and increasing concentrations of unlabeled chemokines. After incubation for 4 h with shaking, the beads were washed three times under vacuum with binding buffer containing 0.15 M NaCl, and the radioactivity was counted after the addition of 50 μl scintillant.
Pharmacokinetic analysis
Three female mice were injected i.v. and s.c. with 0.5, 2, and 8 mg/kg P8A-CCL2 or WT CCL2. The mice were killed at the time-points indicated (see Fig. 3, B and C), and the concentration of CCL2 proteins in the serum was determined using an ELISA for human CCL2 (R&D Systems, Minneapolis, MN, USA), according to the manufacturer’s instructions. The concentration of JE was determined in a similar manner by ELISA (R&D Systems).
Receptor down-modulation and recycling in vitro and in vivo
Freshly isolated murine splenocytes were incubated for 45 min at 37°C with various concentrations of WT CCL2 or P8A-CCL2 diluted in PBS. To measure down-modulation of CCR2, cells were placed on ice and stained immediately for surface expression of CCR2 as described below. To measure recycling of CCR2, cells were washed three times with ice-cold PBS, further incubated at 37°C in PBS for various periods of time, and then stained for surface expression of CCR2 as described below. For down-modulation and recycling of CCR2 in vivo, C57BL/6 mice were injected i.p. with 0.5 and 3 mg/kg P8A-CCL2 in 200 μl PBS. Blood was drawn prior to the administration of the proteins and at 1 h and 4 h after injection, incubated with 2 mM EDTA and stained immediately for surface expression of CCR2 as described below.
Surface expression of CCR2 on splenocytes or full blood was determined by staining the cells for 1 h on ice with 10 μg/ml anti-murine CCR2 antibody, MC-21 [26], or the isotype control antibody, rat IgG2b (BD PharMingen, San Diego, CA, USA; clone R35-38). Samples were subsequently incubated with 5 μg/ml biotinylated anti-rat-IgG2b antibody (BD Biosciences, San Jose, CA, USA; clone RG7/11.1) for 45 min, followed by incubation with 10% rat serum for 10 min and a final incubation with Streptavidin-PE (Dako Cytomation, Carpinteria, CA, USA), anti-CD11b-FITC (BD Bioscience, clone M1/70), and anti-Gr-1-allophycocyanin (BD Bioscience, clone RB6-8C5). RBCs were lysed with FACS-lysing solution (BD Biosciences) and analyzed on a FACSCalibur with CellQuest analysis software. CD11b+ Gr-1+ monocytes were gated, and their surface CCR2 expression was determined as follows: [CCR2 mean fluorescence (P8A)–isotype control mean fluorescence (PBS)]/[CCR2 mean fluorescence (PBS)–isotype control mean fluorescence (PBS)].
Surface expression of CCR2 on THP-1 cells following down-regulation and recycling, performed as described above for murine monocytes, was determined by the binding of radiolabeled CCL2. The cells (1×105 per well in 50 mM Tris-HCl, pH 7.5, containing 1 mM CaCl2, 5 mM MgCl2, and 0.5% BSA) were incubated in 96-well filter plates with 0.1 nM 125I-CCL2 for 1 h at room temperature. After washing three times with the same buffer, supplemented with 0.5 M NaCl, the radioactivity was counted (Wallac, Waltham, MA, USA) after the addition of 50 μl scintillant.
In vitro chemotaxis
Chemotaxis was carried out using THP-1 cells as described previously [20]. Receptor down-modulation was performed by incubating the cells with 0.1 μg P8A-CCL2 or CCL2 for 45 min at 37°C, and recycling was carried out as described above. Two down-modulation experiments were performed in parallel to enable the analysis of their chemotactic response to P8A-CCL2 or CCL2 at the times indicated (see Fig. 5A).
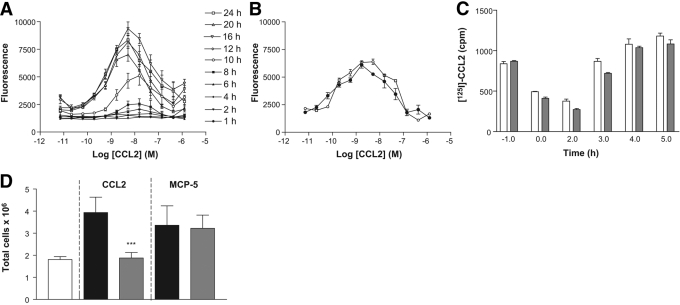
Fig. 5.
Chemotactic response following receptor recycling. (A) Induction of CCR2 down-modulation was induced in THP-1 cells with 0.1 μg P8A-CCL2, and the receptor was then allowed to recycle. Chemotaxis was determined as described in the text at the times indicated. The results are representative of one of two experiments. (B) The chemotactic response of THP-1 cells to CCL2 was determined at Time 0 (•) and after 24 h (○) in culture without prior stimulation. The results are representative of one of two experiments. (C) THP-1 cells were incubated with 0.1 μg/ml CCL2 (white bars) or P8A-CCL2 (gray bars) for 45 min at 37°C. The supernatant was removed to allow the receptor to recycle. Cell-surface receptor was determined by incubation of the cells with [125I]-CCL2 for 1 h as described in the text. The results are representative of one of two experiments. (D) P8A-CCL2 or saline control was administered i.p. 30 min prior to 0.5 mg/kg CCL2 or MCP-5, and the cells enumerated following peritoneal lavage as described in the text after 18 h. The white bar is the saline control; black bars represent the maximal response to CCL2 or MCP-5 using saline 30 min prior to CCL2 or MCP-5 administration; gray bars show the effect of administration of P8A-CCL2 30 min prior to CCL2/MCP-5 administration. Significance is indicated by ***, P < 0.001, when comparing with NaCl-treated animals.
Statistical tests
Statistically significant inhibition of cell recruitment was tested by one-way ANOVA, with a Bonferroni post-test to compare each treatment with vehicle-treated group. Levels of significance were assigned as follows: *, P < 0.05; **, P < 0.01; ***, P < 0.001.
RESULTS
Cellular recruitment in vivo
In previous studies, we observed that P8A-CCL2 as well as monomeric variants of RANTES/CCL5 and MIP-1β/CCL4 were unable to induce cellular recruitment into the peritoneal cavity of Balb/c mice despite their ability to induce cell migration in vitro as effectively as their WT counterparts. As a further test of the importance of oligomerization, here, we investigated whether P8A-CCL2 was able to inhibit WT CCL2-induced recruitment of cells. In the peritoneal assay, P8A-CCL2 inhibited WT CCL2 in a dose-dependent manner with maximal inhibition observed at 0.5 mg/kg (Fig. 1A). Similarly, using the nonspecific stimulus, thioglycollate, previously shown to involve CCL2 and CCR2 in cell recruitment [26, 27], dose-related inhibition was also observed, albeit with less potency, showing maximal inhibition at 10 mg/kg (Fig. 1B). Finally, in a third model involving OVA-induced recruitment into the BAL fluid, P8A-CCL2 inhibited recruitment by 69% at a dose of 0.5 mg/kg, whereas the 44ANAA47-CCL5 variant, previously shown to have antagonistic properties [21], was less effective at the same dose, showing a reduction of 56% (Fig. 1C). The results demonstrate unequivocally that the monomeric chemokine variant has anti-inflammatory properties.
EAE
We next tested P8A-CCL2 in two similar models of MOG-induced EAE. The first model used two sensitizations with MOG, and the second had one. The first experiment with the double-sensitization procedure resulted in mild disease with a maximal clinical score of 2, and the treatment with P8A-CCL2 at three daily doses, 0.15, 0.5, and 1.5 mg/kg, caused a highly significant decrease in clinical score (Fig. 2A). Consistently, histopathology of brain and spinal cord showed a reduction in inflammation and axonal loss (results not shown). In a second experiment, where the doses were reduced in view of the first results, the disease was more severe, reaching a clinical score of 3.5, and P8A-CCL2 showed dose-related activity but only reached significance at the highest dose tested, 0.5 mg/kg (Fig. 2B). Note that the differences in disease severity between the two experiments are commonly observed in EAE, and such variation has been hypothesized to be a result of seasonal changes [28]. Using the single sensitization protocol, prophylactic and therapeutic regimens were tested for 5 days using a dose of 1.5 mg/kg/day. As shown in Figure 2C, treatment starting on Day 10 at the onset of disease symptoms was effective in preventing disease, as the clinical score only reached 1 instead of 3. The curative treatment starting on Day 13 when the disease score was >2 resulted in a decrease in disease score to <1.5. Lastly, the monomeric variant and the WT CCL2 proteins were tested in the same EAE experiment at a dose of 1.5 mg/kg/day. As shown in Figure 2D, no reduction in clinical score was observed for the WT protein in comparison with the significant reduction with P8A-CCL2, again illustrating the anti-inflammatory properties of the monomeric variant.
Systemic administration of P8A-CCL2 displaces the murine MCP-1 homologue, JE
To begin to understand the mechanism for the anti-inflammatory properties of P8A-CCL2, several experiments were conducted. As for WT CCL2, P8A-CCL2 is able to bind GAGs, albeit with slightly reduced affinity [20, 29]. However, it is unable to oligomerize in solution by itself or in the presence of GAGs [22]. We therefore investigated whether it was able to oligomerize with radiolabeled WT CCL2 immobilized on heparin. As shown in Figure 3A, although competition with increasing amounts of unlabeled WT CCL2 results in an increase in radioactivity indicative of oligomerization, there is no increase when unlabeled P8A-CCL2 is used, confirming that although it binds heparin [29], it does not oligomerize, even when WT CCL2 is present.
Because of the difference in the behavior of WT and P8A-CCL2 in binding to GAGs, we next investigated whether s.c.-administered P8A-CCL2 might show a faster rate of appearance in the blood, thereby causing inhibition by flooding the peripheral blood with a protein capable of signaling and receptor down-regulation. The plasma exposure of P8A-CCL2 was determined using an ELISA for CCL2 with three doses of protein, 0.5, 2, and 8 mg/kg, administered i.v., i.p (results not shown), and s.c. (Fig. 3B). Plasma exposure was essentially the same by the i.p. and s.c. route, and maximal exposure occurred at ∼30 min, and the half-life calculated using i.v. administration was determined to be 1–3 h. However, similar results were observed for WT CCL2 (not shown), ruling out this hypothesis.
As the variant binds GAGs, we also postulated that it could displace the endogenous JE. As shown in Figure 3C, JE was displaced in a dose-dependent manner with maximal detection at 2 h. As the monomeric P8A-CCL2 has been shown to be unable to recruit cells in vivo, the displacement of the oligomeric WT protein from the endothelial surface and replacement with the monomeric variant would thus prevent further cell recruitment. This hypothesis was substantiated further in a peritoneal recruitment experiment induced by thioglycollate, where a single administration of P8A-CCL2 was given at different time-points ranging from 15 min to 72 h prior to the thioglycollate stimulus (Fig. 3D). Although the extent of inhibition was less than that observed when a second administration was given 24 h after the thioglycollate stimulus, it was similar when P8A-CCL2 was given 15 min or 24 h prior to the stimulus but lost at the –48 h and –72 h time-points, clearly demonstrating immobilization of the protein, presumably on the endothelial surface.
Down-modulation and recycling of CCR2
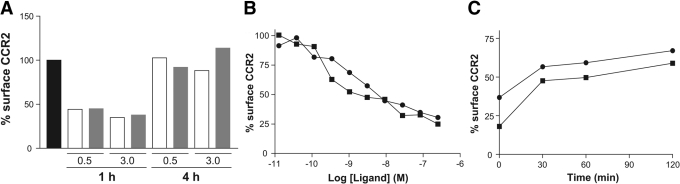
As P8A-CCL2 retains full receptor activation capacity [22], we investigated its ability to cause down-modulation of CCR2 in vivo and in vitro. P8A-CCL2 was administered i.p to mice at doses of 0.5 and 3 mg/kg. Surface CCR2 on circulating granulocytes was determined using MC-21 [26]. In two separate experiments, at both doses tested, ∼60% down-modulation was observed after 1 h (Fig. 4A), which is similar to that observed in vitro. After 4 h, the surface receptor level had returned to the original level following down-modulation with P8A-CCL2 and WT CCL2 (Fig. 4A). As shown in Figure 4B, P8A-CCL2 induces the down-modulation of the receptor on murine monocytes in vitro in a dose-related manner after 30 min incubation with identical potency to WT CCL2. When the ligand is removed from the culture supernatant, the receptor recycles to the cell surface (Fig. 4C).
Fig. 4.
CCR2 down-regulation and recycling in vivo and in vitro. (A) Internalization of CCR2 on murine monocytes was determined by FACS analysis of GR1+ monocytes with an anti-mCCR2 antibody after i.p. administration of 0.5 and 3 mg/kg CCL2 (open bars) or P8A-CCL2 (gray bars). The surface expression of CCR2 prior to administration of the proteins is shown in the black bar. The results are representative of one of two experiments. (B) CCR2 down-regulation in vitro was determined by FACS analysis after incubation of purified GR1+ monocytes with CCL2 (•) or P8A-CCL2 (▪) at 37°C for 30 min. (C) Recycling of CCR2 as determined by FACS analysis after removal of CCL2 (•) or P8A-CCL2 (▪) from the culture supernatant. The results are representative of one of three experiments.
To assess the functionality of CCR2 after recycling, a second set of experiments was performed with THP-1 cells. Receptor down-modulation was induced with 0.1 μg/ml P8A-CCL2 or WT CCL2, and recycling was allowed to proceed for 2 h, after which, the cells were assessed for their ability to migrate in a chemotaxis chamber. Although CCR2 had been shown to recycle to the cell surface of murine monocytes attaining 100% of the original receptor population after 4 h in vivo and 70% after 2 h in vitro (Fig. 4, A and C), the cells showed no migratory response until 8–10 h (Fig. 5A). The cells were also tested in the chemotaxis assay without receptor down-modulation at the beginning of the experiment and after 24 h (Fig. 5B). To determine whether the kinetics of restoration of the ability of the receptor to cause chemotaxis correlates with ligand binding, down-modulation and recycling were repeated, and the cells were analyzed for their ability to bind radiolabeled CCL2. As shown in Figure 5C, the capacity to bind CCL2 was restored fully at ∼3 h, well before full capacity to cause cell migration. Lastly, the effect of receptor down-modulation as a potential inhibitory mechanism of action of P8A-CCL2 was tested in vivo in the peritoneal cavity by inducing cellular recruitment with another specific CCR2 ligand, MCP-5. As shown in Figure 5D, P8A-CCL2 was only able to inhibit CCL2 recruitment but had no effect on that induced by MCP-5.
Intravital microscopy
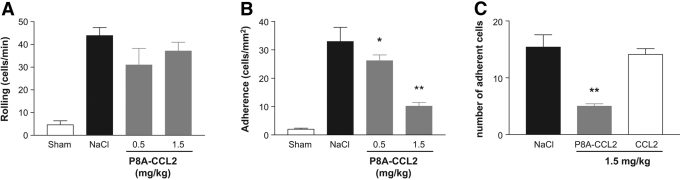
To further ascertain differences between P8A-CCL2 and WT CCL2 that might explain the mechanism of action for the inhibitory behavior of P8A-CCL2, we examined the effects of both proteins on recruitment of cells into the brain of mice that had been subjected to induction of EAE using the single immunization protocol. As shown in Figure 6B, P8A-CCL2 showed a dose-related ability to inhibit adhesion of cells to pial vessels, and it had no effect on rolling (Fig. 6A). However, WT CCL2 had no effect on cellular adhesion at doses of 1.5 mg/kg (Fig. 6C) and 5 mg/kg (not shown).
Fig. 6.
P8A-CCL2 but not CCL2 prevents leukocyte adhesion in the brain microvasculature. Intravital microscopy was used to assess the rolling and firm arrest of leukocytes on pial microvessels. P8A-CCL2 (0.5 or 1.5 mg/kg, s.c.) was administered on Day 14 after EAE induction, and (A) leukocyte rolling and (B) adhesion on pial vessels were quantified after 45 min. Control animals received vehicle (200 μl saline). (C) Leukocyte adhesion was quantified 45 min after administration of P8A-CCL2 or CCL2 (both at 1.5 mg/kg, s.c.). The number of animals in each experimental group was ≥5, and results are shown as the mean ± sem. Significance is indicated by *, P < 0.05, and **, P < 0.01, when comparing with NaCl-treated animals.
DISCUSSION
CCL2 has been shown by many laboratories to be important in human disease as well as in animal models. Several approaches have validated the CCL2-CCR2 arm in disease models, including ligand and receptor gene knockouts [6,7,8, 27], neutralizing anti-CCL2 [9] and anti-CCR2 antibodies [10], modified CCL2 receptor antagonists [11, 13], and low molecular weight inhibitors of CCR2 [30], as well as inhibitors interfering with the CCL2 pathway through an unidentified mechanism [14]. We have demonstrated previously that P8A-CCL2 is an obligate monomer that is unable to dimerize [22] and moreover, is unable to form higher order aggregates with the murine ortholog, JE, as assessed by size exclusion chromatography (results not shown). In this work, we discovered yet another molecular approach for inhibiting inflammation, linked to the requirement for oligomerization of CCL2 for activity in vivo [20]. Importantly, the anti-inflammatory properties of P8A-CCL2 are not restricted to the disease model reported here; P8A-CCL2 has also been shown to reduce symptoms in another rodent model, adjuvant induced arthritis in the rat [31]. However, in both cases, these properties were quite surprising, and the mechanism of action was not predicted rationally or easily explained.
We previously created a variant of CCL5 that was impaired in its ability to bind GAGs and showed potent anti-inflammatory properties in EAE. Experiments aimed at investigating its mechanism of action suggest that it functions through a dominant-negative mechanism by sequestering WT CCL5 into an inactive heterodimeric form [21]. As P8A-CCL2 cannot form dimers, this explanation is not possible for this variant. However, the possibility that it competes for GAG-binding sites was tested, and the experimental evidence reported here substantiates this as a contributing mechanism.
Densensitization was also hypothesized as a potential mechanism. To this end, we speculated that WT CCL2 administered systemically could have the same effect. However, as shown by the intravital microscopy study, the effects of the two proteins differed: P8A-CCL2 inhibited cell adhesion to vessel surfaces by mechanisms that remain to be determined, and WT CCL2 did not. Moreover, we were not able to demonstrate a reduction in clinical score with WT CCL2 in EAE models, whereas P8A-CCL2 was effective. However, studies about the possibility of desensitization as the mechanism of action revealed an interesting phenomenon. After down-modulation and recycling of CCR2, there is a considerable lag period before the cells regain functionality. This does not appear to be a result of reconstitution of the active conformation of the receptor, as binding of the ligand appears to be unaffected at early time-points after receptor recycling. It appears that this defect may be attributable to the reconstitution of the signaling machinery, a phenomenon that requires further studies. We also noted that P8A-CCL2 inhibited migration to CCL2 but not MCP-5, another CCR2 ligand; as CCR2 is the only known receptor of MCP-5, this observation also requires further investigation.
In summary, we describe an alternative to the well-known strategy of making N-terminal modifications to chemokines that produce antagonists [32,33,34], which has the advantage of retaining CCR2 activation for the maintenance of the adaptive immune response, where abrogation of CCR2 activity has been shown to be deleterious [35]. However, the combination of abrogation of oligomerization with N-terminal modifications could also be envisaged, which may produce exceptionally powerful, anti-inflammatory protein therapeutics that exert their effects via a combination of several mechanisms, including the ability to antagonize and the inability to oligomerize.
Acknowledgments
This work was supported by National Institutes of Health grant NIH RO1-AI37113 to T. M. H. and the European Union FP6 (INNOCHEM, grant number LSHB-CT-2005-518167).
References
- Luster A D. Chemokines–chemotactic cytokines that mediate inflammation. N Engl J Med. 1998;338:436–445. doi: 10.1056/NEJM199802123380706. [DOI] [PubMed] [Google Scholar]
- Handel T M, Johnson Z, Crown S E, Lau E K, Proudfoot A E. Regulation of protein function by glycosaminoglycans—as exemplified by chemokines. Annu Rev Biochem. 2005;74:385–410. doi: 10.1146/annurev.biochem.72.121801.161747. [DOI] [PubMed] [Google Scholar]
- Rollins B J. Monocyte chemoattractant protein 1: a potential regulator of monocyte recruitment in inflammatory disease. Mol Med Today. 1996;2:198–204. doi: 10.1016/1357-4310(96)88772-7. [DOI] [PubMed] [Google Scholar]
- Proudfoot A E. Chemokine receptors: multifaceted therapeutic targets. Nat Rev Immunol. 2002;2:106–115. doi: 10.1038/nri722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity. 2000;12:121–127. doi: 10.1016/s1074-7613(00)80165-x. [DOI] [PubMed] [Google Scholar]
- Boring L, Gosling J, Cleary M, Charo I F. Decreased lesion formation in CCR2(−/−) mice reveals a role for chemokines in the initiation of atherosclerosis. Nature. 1998;394:894–897. doi: 10.1038/29788. [DOI] [PubMed] [Google Scholar]
- Izikson L, Klein R S, Charo I F, Weiner H L, Luster A D. Resistance to experimental autoimmune encephalomyelitis in mice lacking the CC chemokine receptor (CCR)2. J Exp Med. 2000;192:1075–1080. doi: 10.1084/jem.192.7.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang D R, Wang J, Kivisakk P, Rollins B J, Ransohoff R M. Absence of monocyte chemoattractant protein 1 in mice leads to decreased local macrophage recruitment and antigen-specific T helper cell type 1 immune response in experimental autoimmune encephalomyelitis. J Exp Med. 2001;193:713–726. doi: 10.1084/jem.193.6.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd C M, Minto A W, Dorf M E, Proudfoot A, Wells T N, Salant D J, Gutierrez-Ramos J C. RANTES and monocyte chemoattractant protein-1 (MCP-1) play an important role in the inflammatory phase of crescentic nephritis, but only MCP-1 is involved in crescent formation and interstitial fibrosis. J Exp Med. 1997;185:1371–1380. doi: 10.1084/jem.185.7.1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath C, Welt F G, Nedelman M, Rao P, Rogers C. Targeting CCR2 or CD18 inhibits experimental in-stent restenosis in primates: inhibitory potential depends on type of injury and leukocytes targeted. Circ Res. 2002;90:488–494. doi: 10.1161/hh0402.105956. [DOI] [PubMed] [Google Scholar]
- Gong J H, Ratkay L G, Waterfield J D, Clark L I. An antagonist of monocyte chemoattractant protein 1 (MCP-1) inhibits arthritis in the MRL-lpr mouse model. J Exp Med. 1997;186:131–137. doi: 10.1084/jem.186.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano K, Egashira K, Ohtani K, Zhao G, Funakoshi K, Ihara Y, Sunagawa K. Catheter-based adenovirus-mediated anti-monocyte chemoattractant gene therapy attenuates in-stent neointima formation in cynomolgus monkeys. Atherosclerosis. 2007;194:309–316. doi: 10.1016/j.atherosclerosis.2006.10.029. [DOI] [PubMed] [Google Scholar]
- Schepers A, Eefting D, Bonta P I, Grimbergen J M, de Vries M R, van Weel V, de Vries C J, Egashira K, van Bockel J H, Quax P H. Anti-MCP-1 gene therapy inhibits vascular smooth muscle cells proliferation and attenuates vein graft thickening both in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2006;26:2063–2069. doi: 10.1161/01.ATV.0000235694.69719.e2. [DOI] [PubMed] [Google Scholar]
- Sironi M, Guglielmotti A, Polentarutti N, Fioretti F, Milanese C, Romano M, Vigini C, Coletta I, Sozzani S, Bernasconi S, Vecchi A, Pinza M, Mantovani A. A small synthetic molecule capable of preferentially inhibiting the production of the CC chemokine monocyte chemotactic protein-1. Eur Cytokine Netw. 1999;10:437–442. [PubMed] [Google Scholar]
- Bhatia M, Ramnath R D, Chevali L, Guglielmotti A. Treatment with bindarit, a blocker of MCP-1 synthesis, protects mice against acute pancreatitis. Am J Physiol Gastrointest Liver Physiol. 2005;288:G1259–G1265. doi: 10.1152/ajpgi.00435.2004. [DOI] [PubMed] [Google Scholar]
- Zoja C, Corna D, Benedetti G, Morigi M, Donadelli R, Guglielmotti A, Pinza M, Bertani T, Remuzzi G. Bindarit retards renal disease and prolongs survival in murine lupus autoimmune disease. Kidney Int. 1998;53:726–734. doi: 10.1046/j.1523-1755.1998.00804.x. [DOI] [PubMed] [Google Scholar]
- Guglielmotti A, D'Onofrio E, Coletta I, Aquilini L, Milanese C, Pinza M. Amelioration of rat adjuvant arthritis by therapeutic treatment with bindarit, an inhibitor of MCP-1 and TNF-α production. Inflamm Res. 2002;51:252–258. doi: 10.1007/pl00000301. [DOI] [PubMed] [Google Scholar]
- Bruhl H, Cihak J, Schneider M A, Plachy J, Rupp T, Wenzel I, Shakarami M, Milz S, Ellwart J W, Stangassinger M, Schlondorff D, Mack M. Dual role of CCR2 during initiation and progression of collagen-induced arthritis: evidence for regulatory activity of CCR2+ T cells. J Immunol. 2004;172:890–898. doi: 10.4049/jimmunol.172.2.890. [DOI] [PubMed] [Google Scholar]
- Haringman J J, Gerlag D M, Smeets T J, Baeten D, van den, Bosch F, Bresnihan B, Breedveld F C, Dinant H J, Legay F, Gram H, Loetscher P, Schmouder R, Woodworth T, Tak P P. A randomized controlled trial with an anti-CCL2 (anti-monocyte chemotactic protein 1) monoclonal antibody in patients with rheumatoid arthritis. Arthritis Rheum. 2006;54:2387–2392. doi: 10.1002/art.21975. [DOI] [PubMed] [Google Scholar]
- Proudfoot A E I, Handel T M, Johnson Z, Lau E K, LiWang P, Clark L, Borlat F, Wells T N C, Kosco-Vilbois M H. Glycosaminoglycan binding and oligomerization are essential for the in vivo activity of certain chemokines. Proc Natl Acad Sci USA. 2003;100:1885–1890. doi: 10.1073/pnas.0334864100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson Z, Kosco-Vilbois M H, Herren S, Cirillo R, Muzio V, Zaratin P, Carbonatto M, Mack M, Smailbegovic A, Rose M, Lever R, Page C, Wells T N, Proudfoot A E. Interference with heparin binding and oligomerization creates a novel anti-inflammatory strategy targeting the chemokine system. J Immunol. 2004;173:5776–5785. doi: 10.4049/jimmunol.173.9.5776. [DOI] [PubMed] [Google Scholar]
- Paavola C D, Hemmerich S, Grunberger D, Polsky I, Bloom A, Freedman R, Mulkins M, Bhakta S, McCarley D, Wiesent L, Wong B, Jarnagin K, Handel T M. Monomeric monocyte chemoattractant protein-1 (MCP-1) binds and activates the MCP-1 receptor CCR2B. J Biol Chem. 1998;273:33157–33165. doi: 10.1074/jbc.273.50.33157. [DOI] [PubMed] [Google Scholar]
- Sahrbacher U C, Lechner F, Eugster H P, Frei K, Lassmann H, Fontana A. Mice with an inactivation of the inducible nitric oxide synthase gene are susceptible to experimental autoimmune encephalomyelitis. Eur J Immunol. 1998;28:1332–1338. doi: 10.1002/(SICI)1521-4141(199804)28:04<1332::AID-IMMU1332>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- dos Santos A C, Barsante M M, Arantes R M, Bernard C C, Teixeira M M, Carvalho-Tavares J. CCL2 and CCL5 mediate leukocyte adhesion in experimental autoimmune encephalomyelitis—an intravital microscopy study. J Neuroimmunol. 2005;162:122–129. doi: 10.1016/j.jneuroim.2005.01.020. [DOI] [PubMed] [Google Scholar]
- Hoogewerf A J, Kuschert G S, Proudfoot A E, Borlat F, Clark-Lewis I, Power C A, Wells T N. Glycosaminoglycans mediate cell surface oligomerization of chemokines. Biochemistry. 1997;36:13570–13578. doi: 10.1021/bi971125s. [DOI] [PubMed] [Google Scholar]
- Mack M, Cihak J, Simonis C, Luckow B, Proudfoot A E, Plachy J, Bruhl H, Frink M, Anders H J, Vielhauer V, Pfirstinger J, Stangassinger M, Schlondorff D. Expression and characterization of the chemokine receptors CCR2 and CCR5 in mice. J Immunol. 2001;166:4697–4704. doi: 10.4049/jimmunol.166.7.4697. [DOI] [PubMed] [Google Scholar]
- Lu B, Rutledge B J, Gu L, Fiorillo J, Lukacs N W, Kunkel S L, North R, Gerard C, Rollins B J. Abnormalities in monocyte recruitment and cytokine expression in monocyte chemoattractant protein 1-deficient mice. J Exp Med. 1998;187:601–608. doi: 10.1084/jem.187.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teuscher C, Doerge R W, Fillmore P D, Blankenhorn E P. eae36, a locus on mouse chromosome 4, controls susceptibility to experimental allergic encephalomyelitis in older mice and mice immunized in the winter. Genetics. 2006;172:1147–1153. doi: 10.1534/genetics.105.049049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau E K, Paavola C D, Johnson Z, Gaudry J P, Geretti E, Borlat F, Kungl A J, Proudfoot A E, Handel T M. Identification of the glycosaminoglycan binding site of the CC chemokine, MCP-1: implications for structure and function in vivo. J Biol Chem. 2004;279:22294–22305. doi: 10.1074/jbc.M311224200. [DOI] [PubMed] [Google Scholar]
- Brodmerkel C M, Huber R, Covington M, Diamond S, Hall L, Collins R, Leffet L, Gallagher K, Feldman P, Collier P, Stow M, Gu X, Baribaud F, Shin N, Thomas B, Burn T, Hollis G, Yeleswaram S, Solomon K, Friedman S, Wang A, Xue C B, Newton R C, Scherle P, Vaddi K. Discovery and pharmacological characterization of a novel rodent-active CCR2 antagonist, INCB3344. J Immunol. 2005;175:5370–5378. doi: 10.4049/jimmunol.175.8.5370. [DOI] [PubMed] [Google Scholar]
- Shahrara S, Proudfoot A E, Park C C, Volin M V, Haines G K, Woods J M, Aikens C H, Handel T M, Pope R M. Inhibition of monocyte chemoattractant protein-1 ameliorates rat adjuvant-induced arthritis. J Immunol. 2008;180:3447–3456. doi: 10.4049/jimmunol.180.5.3447. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Rollins B J. A dominant negative inhibitor indicates that monocyte chemoattractant protein 1 functions as a dimer. Mol Cell Biol. 1995;15:4851–4855. doi: 10.1128/mcb.15.9.4851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Proudfoot A E I, Power C A, Hoogewerf A J, Montjovent M O, Borlat F, Offord R E, Wells T N C. Extension of recombinant human RANTES by the retention of the initiating methionine produces a potent antagonist. J Biol Chem. 1996;271:2599–2603. doi: 10.1074/jbc.271.5.2599. [DOI] [PubMed] [Google Scholar]
- Gong J H, Clark-Lewis I. Antagonists of monocyte chemoattractant protein 1 identified by modification of functionally critical NH2-terminal residues. J Exp Med. 1995;181:631–640. doi: 10.1084/jem.181.2.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serbina N V, Pamer E G. Monocyte emigration from bone marrow during bacterial infection requires signals mediated by chemokine receptor CCR2. Nat Immunol. 2006;7:311–317. doi: 10.1038/ni1309. [DOI] [PubMed] [Google Scholar]