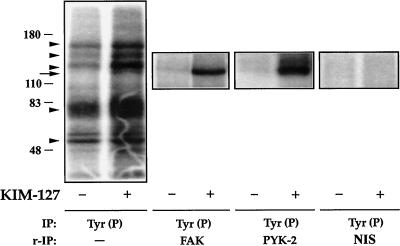
Figure 4.
Presence of FAK and PYK-2 in anti-Tyr(P) immunoprecipitates from T cells after activation of LFA-1. T lymphoblasts (50 × 106 cells) plated on ICAM-1-Fc–coated plastic dishes were allowed to adhere for 30 min in the absence (−) or presence (+) of 10 μg/ml mAb KIM-127. The cells were then lysed, the extracts were immunoprecipitated (IP) with the PY20 anti-Tyr(P) mAb [IP: Tyr(P)], and kinase reactions were performed as described in MATERIALS AND METHODS. In parallel cultures of lymphoblasts, which were allowed to adhere to ICAM-1-Fc and stimulated or not with KIM-127 as above, after the kinase reactions were carried out, the major Tyr(P)-labeled bands were eluted from the PY20 immunocomplexes by boiling the pellet in 100 μl of a solution containing 10 mM Tris, pH 7.4 and 1% SDS. Denatured Tyr(P) proteins were then reimmunoprecipitated (r-IP) with the anti-FAK C-20 to confirm the presence of FAK [IP: Tyr(P) and r-IP: FAK], with the anti-PYK-2 C-19 to confirm the presence of PYK-2 [IP: Tyr(P) and r-IP: PYK-2], or with nonimmune rabbit serum control [IP: Tyr (P) and r-IP: NIS]. After SDS-PAGE of PY20 immunoprecipitates and C-20 and C-19 reimmunoprecipitates, gels were subjected to autoradiography to detect total phosphotyrosines FAK and PYK-2, respectively. The position of the major phosphorylated bands in the gel is indicated with arrowheads. The FAK and PYK-2 position is indicated by an arrow. Molecular mass markers (in kilodaltons) are shown on the left. The results are representative of three independent experiments.