Abstract
Recent studies suggest that activation of the peripheral immune system elicits a discordant central (i.e., in the brain) inflammatory response in aged but otherwise healthy subjects compared with younger cohorts. A fundamental difference in the reactive state of microglial cells in the aged brain has been suggested as the basis for this discordant inflammatory response. Thus, the aging process appears to serve as a “priming” stimulus for microglia, and upon secondary stimulation with a triggering stimulus (i.e., peripheral signals communicating infection), these primed microglia release excessive quantities of proinflammatory cytokines. Subsequently, this exaggerated cytokine release elicits exaggerated behavioral changes including anorexia, hypersomnia, lethargy, decreased social interaction, and deficits in cognitive and motor function (collectively known as the sickness behavior syndrome). Whereas this reorganization of host priorities is normally adaptive in young subjects, there is a propensity for this response to be maladaptive in aged subjects, resulting in greater severity and duration of the sickness behavior syndrome. Consequently, acute bouts of cognitive impairment in elderly subjects increase the likelihood of poor self-care behaviors (i.e., anorexia, weight loss, noncompliance), which ultimately leads to higher rates of hospitalization and mortality.
Keywords: cytokine, inflammation, microglia, sickness behavior
INTRODUCTION
Although the immune system and brain are in constant communication, transfer of information between these systems increases during peripheral infection. Upon stimulation of the innate immune system, brain microglial cells react to signals from the periphery and produce inflammatory cytokines to coordinate a complementary behavioral response that is normally adaptive [1]. Excessive production of proinflammatory cytokines in the brain, however, can produce severe behavioral deficits and promote neurotoxicity. For instance, in the murine ME7 model of prion disease, administration of lipopolysaccharide (LPS) to activate the peripheral innate immune system induces an aberrant proinflammatory cytokine response by brain microglial cells, elicits excessive sickness behavior, and accelerates progression of the neurodegenerative disease [2, 3]. A similar interaction between peripheral infection and other chronic neurodegenerative diseases has also been reported in rodent models [4]. Therefore, it was proposed that elements unique to the neurodegenerative disease provide a priming or sensitizing stimulus for microglia, while subsequent signals from peripheral immune stimulation provide a secondary triggering stimulus [5]. The result of combining the priming and triggering stimuli is an overall response that is greater in magnitude than the sum of responses to individual stimuli alone. This interaction may help to explain why infection serves as a risk factor for relapse in multiple sclerosis patients or dementia in patients with Alzheimer’s disease [6, 7].
Similar to chronic neurodegenerative diseases, aging was recently proposed to prime microglial cells [8,9,10]. Specifically, major histocompatibility complex (MHC) class II, a marker for activated microglia, was increased in the brain of healthy aged mice [10] and i.p. administration of LPS resulted in an exaggerated inflammatory cytokine response in the aged brain [10, 11]. Similar findings have been reported in older rats inoculated with Escherichia coli [12], and the discordant cytokine response in the brain was shown to be separate from peripheral immune signals, because intracerebroventricular injection of LPS elicited the same discordant response in aged mice [13]. Consistent with an exaggerated proinflammatory cytokine response in the brain, aged mice were shown to exhibit more severe anorexia, depression-like behaviors, and deficits in hippocampal-dependent learning and memory compared with younger cohorts after i.p. LPS injection [10, 11, 14].
These observations from rodent studies are noteworthy because acute cognitive impairments are common in elderly human patients and often occur in association with peripheral infection [15, 16]. Moreover, demented elderly patients are routinely screened for bladder infections because of the close association between infection and cognitive disorders. It is also interesting that elderly patients suffering from pneumonia often present symptoms consistent with delirium [17]. Thus, an inflammatory response initiated during infection that is normally adaptive may elicit severe behavioral deficits in elderly individuals, ultimately leading to prolonged recovery periods. How aging affects the central cytokine response and subsequently behavior is critical given the rapid growth of the human population aged 65 years or older. Therefore, the purpose of this review is to explain microglial cell priming, discuss evidence suggesting a link between normal aging and neuroinflammation, and detail several discordant responses that result when physiological processes turn pathological in the aged brain.
Cross-talk between the immune system and brain
While central neurons and innate immune cells (e.g., microglia, astrocytes, etc.) possess toll-like receptors capable of directly detecting bacteria and viruses [18, 19], it is interesting that the discrete presence of foreign pathogens in the periphery still causes a sickness behavior syndrome in animals that includes, e.g., fever, anorexia, and lethargy. Because peripheral innate immune cells also have receptors capable of detecting these noncognitive stimuli, how the immune system transmits a message to the brain has been studied to understand why behavior is altered in sick animals. Somehow, the brain must sense, process, and coordinate peripheral signals to maintain homeostasis during infection. A major dilemma to understanding how peripheral immune signals access the brain has been determining the role of the blood-brain barrier (BBB), since the BBB is a physical impediment that restricts the movement of humoral molecules. Indeed, the brain was traditionally considered an “immune-privileged” site due to its seclusion by the BBB. This dilemma has been partially solved, as at least four mechanisms by which the immune system communicates with the brain have been described, and it now appears that the brain forms a representation of the peripheral immune status with only minimal direct contact [20]. The signals necessary to convey this information originate with inflammatory mediators (i.e., cytokines) by virtue of their type and concentration. While discussed here in brief, the reader is referred to the elegant review of brain-immune communication pathways by Quan and Banks [21] for more detailed information.
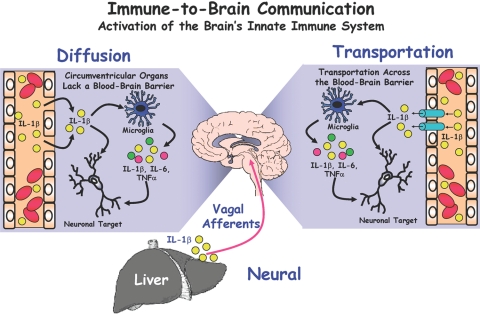
Figure 1 depicts the redundant pathways by which the peripheral immune system communicates with the brain. First, there is good evidence to suggest that cytokines can passively diffuse from the blood into the brain via circumventricular organs [22, 23], which are specialized regions lacking a contiguous BBB. These circumventricular organs are located in close proximity to the hypothalamus, which is a key area for maintaining homeostasis. Thus, entry of cytokines into the central nervous system (CNS) allows the peripheral signals to act directly on hypothalamic neurons involved in thermoregulation, neuroendocrine secretions, and behavior. Perhaps more importantly, however, is the propensity for these peripheral cytokines to induce production of proinflammatory mediators by microglia. Second, in brain regions containing an intact BBB, there is compelling evidence to suggest an energy-dependent, saturable, carrier-mediated transport system for cytokines. To provide evidence of such a pathway, Banks and Kastin [24] intravenously injected radiolabeled, recombinant human IL-1α into rodents and were able to detect both radioactivity and intact IL-1α protein within the CNS parenchyma. Since this initial discovery, active transport systems integral in the BBB have been characterized for IL-1, IL-6, and TNF [24,25,26]. One limitation of this transport system is that putative transporter proteins are yet undefined, and it remains unclear whether the quantity of cytokines transported via this mechanism is actually sufficient to mediate behavioral responses. Third, endothelial cells forming the BBB are themselves capable of secreting immune-related molecules (including NO, prostaglandins, IL-1, and IL-6) when stimulated by peripheral signals [27]. In this scenario, circulating cytokines bind to specific endothelial receptors which, in turn, initiate release of one or more cytokines into the CNS without physical entry of the blood-derived constituents. Recent evidence for this pathway comes from studies wherein the expression of endothelial IL-1 type 1 receptors was specifically knocked-down in mice [28]; this research concluded that IL-1 administered by intravenous, intracerebroventricular, or intraperitoneal routes at least partially required the presence of IL-1 type 1 receptors on endothelial cells to elicit sickness behavior responses.
Fig. 1.
Parallel communication routes between the immune system and brain. The neural pathway involves rapid communication of immune signals via primary afferent nerves. For instance, peripherally produced cytokines directly stimulate vagal afferents from the liver to elicit central responses. In the diffusion pathway, blood-derived cytokines passively traverse the blood-brain barrier at “leaky” brain regions, including the circumventricular organs. Transportation of peripheral cytokines occurs by an energy-dependent, saturable process involving transporters integral in the blood-brain barrier. A fourth route of communication currently being investigated involves endothelial cells composing the blood-brain barrier. It is believed that circulating cytokines bind to endothelial receptors to elicit the release of immune molecules (e.g., cytokines, NOS, etc.) directly into brain regions. Regardless of the pathway, inflammatory mediators released into the CNS activate brain microglial cells to release inflammatory cytokines, which subsequently bind neuronal receptors in specific brain regions (e.g., hippocampus) to initiate the sickness behavior syndrome.
The three aforementioned routes of communication all require blood-derived stimuli (e.g., cytokines) to increase cytokine concentrations in the CNS and ultimately elicit behavioral responses. The fourth known route of neuroimmune communication is different because it involves transmission of peripheral immune signals via the autonomic nervous system (i.e., via the vagus nerve). Peripheral cytokines are thought to directly stimulate vagal afferents which, in turn, rapidly activate central pathways involving sickness behavior [29, 30]. Bluthe et al. [31] demonstrated that sectioning of the vagus nerve was sufficient to block communication between the periphery and brain and thus, to mitigate centrally-mediated LPS-induced sickness behavior. Whereas the central response to LPS administration was blocked by subdiaphragmatic vagotomy, the peripheral immune response was unabated, and peripheral blood cytokine concentrations were elevated as expected. Substantial evidence has now been amassed to support the essential role of vagal afferents in rapid communication of peripheral signals to the brain. Supporting this essential role are studies confirming the presence of IL-1 receptors on vagal paraganglia in close proximity to hepatic and lymph tissue [32] and the observation of timely expression of both IL-1 and IL-1 receptors on vagal paraganglia upon intraperitoneal injection of LPS [33].
Overall, immune-to-brain communication involves a complex and somewhat redundant system that requires careful coordination. It appears that activation of the rapid afferent neural pathway precedes the slower signaling of the cytokine-dependent humoral pathway [1], but ultimately, these convergent pathways culminate in production of inflammatory cytokines by microglial cells in the brain (i.e., neuroinflammation). This point is extremely important, because it appears to be the presence of central cytokines that relays a peripherally derived immune signal to neurons within the CNS. Thus, regardless of where central cytokines originate, it is the direct interaction of cytokines with their neuronal targets that elicits behavioral modifications in the brain. It is still unclear whether peripherally or centrally derived cytokines play a larger role in eliciting central responses, but it may be speculated that reactive microglia in the brain have the potential to release greater quantities of cytokines in a more rapid fashion than peripheral cytokine can enter the brain simply due to the proximity and reactivity of activated brain microglial cells. Regardless, microglial cells and the cytokines they produce are key components of both the central and overall immune response, and mounting evidence suggests that dysregulation of microglial cell activity may be responsible for a discordant central response to peripheral immune signals.
Normal aging as a microglial priming factor
Recent evidence suggests the aged brain resides in a chronic state of neuroinflammation, characterized by increased reactivity upon immune stimulation and low-level production of central cytokines. One working hypothesis suggests that this hyper-reactivity is due to priming of brain microglial cells, which parallels circumstances known to occur in peripheral macrophages. In the classic sense, activation of macrophages requires both a “priming” stimulus (i.e., IFN-γ [34]) and a secondary “triggering” stimulus (e.g., LPS) [34, 35]. The priming effect of IFN-γ is required to cause a phenotypic shift toward a more sensitized state characterized by increased expression of cell-surface antigens, including MHC class II. This “primed” macrophage will then respond to a secondary “triggering” stimulus more rapidly and to a greater degree than would be expected from a nonprimed macrophage [36]. Recently, this priming concept has been applied to brain microglial cells due to an observation that peripheral infection elicits exacerbated inflammatory responses in models of neurodegenerative disease. In such disease states, the effects of a discordant central inflammatory response can be devastating due to severe behavioral deficits and neurotoxicity caused by excessive cytokine release in the brain.
Microglial cells residing in a state of heightened reactivity were first suggested as being associated with neurodegenerative diseases, including multiple sclerosis, Alzheimer’s disease, and the murine ME7 model of prion disease [37]. Activation of the peripheral innate immune system by LPS in the murine prion model caused a discordant release of cytokines by brain microglia, excessive sickness behavior, and rapid progression of the neurodegenerative disease. In this case, a component of the prion disease presumably served to prime microglial cells, and only after LPS administration (i.e., the triggering stimulus) were excessive quantities of IL-1β released [2, 38]. A similar parallel between hyper-responsive microglia and chronic LPS administration was drawn while studying another chronic neurodegenerative disease, amyotrophic lateral sclerosis. Nguyen et al. [4] concluded that chronically activated microglial cells were responsible for the exacerbated disease progression and progressive motor axon degeneration in a transgenic mouse model expressing a mutant form of superoxide dismutase 1. Thus, it appears that one or more unknown elements unique to various neurodegenerative diseases provide a priming stimulus to brain microglia. However, it is possible that other immune effector cells play a role in priming microglial cells.
Using a model of kainic acid-induced seizures, Somera-Molina et al. [39] reported evidence of chronic glial activation in the hippocampus of rats following a rise in proinflammatory cytokines (namely IL-1β from microglia and S100B from astrocytes) early in life. Apart from the potential role of astrocytes to directly prime microglia, it may be speculated that a loss of inhibition by astrocytes or other immune effector cells underlies the occurrence of primed microglia. Dendritic cells and T lymphocytes have also been implicated in eliciting heightened reactivity of microglia and astrocytes [40], and mounting evidence suggests T cells (especially CD8+ T cells) may play a larger role than previously suspected in regulating central immune function [41]. Although evidence suggesting a specific route of microglial priming in the aged brain has remained somewhat elusive, lessons can undoubtedly be learned from studies of neurodegenerative diseases. Namely, subsequent stimulation of the peripheral immune system causes primed microglial cells to be triggered, which culminates in a central response greater in both magnitude and duration than what would be predicted from classically activated microglia [5, 7]. Thus, microglia in subjects with neurodegenerative disease have undergone a phenotypic shift toward a primed or more sensitized state. These microglia chronically express low levels of proinflammatory cytokines, but they are similar to classically activated microglia in terms of morphology and cell-surface antigen expression.
Under normal circumstances, nonprimed microglial cells are transformed into activated immune cells upon stimulation of the innate immune system, with concomitant changes in distribution, morphology, immunophenotype, and metabolism. Swelling of the microglial cell body, a thickening of the proximal processes, and a reduction in the number and complexity of distal processes are key morphological signs of this activation [42, 43]. Additionally, cell surface expression patterns are altered such that CD45, CD68, complement receptors, TLR, and MHC class I and II are up-regulated. Ultimately, these activated microglia release proinflammatory mediators in the brain which, in turn, elicit behavioral modifications designed to speed recovery from sickness [44]. Overall, this innate immune response is designed to be rapid and reversible, although the regulated fate of activated microglial cells remains a contentious point (i.e., whether activated microglia remaining after removal of the foreign insult are able to return to a ramified resting state or whether these microglia undergo apoptosis [45, 46]). Primed microglia are also activated upon stimulation of the innate immune system, but the end result is a discordant release of central cytokines, which changes the course of action from physiological to pathological. It is important to note that cell-surface antigen expression provides key indicators to distinguish nonprimed and primed microglial cells.
Paralleling the phenotypic alterations of microglia that occur in neurodegenerative diseases, the normal aging process may constitute a physiological event associated with increased incidence of primed microglia [9, 10, 37], as depicted in Fig. 2. Evidence suggests that aged, but otherwise healthy, brains of animal and humans exhibit gene expression profiles indicative of increased microglial cell activation and neuroinflammation [9, 10, 47,48,49]. As such, microglia in the brain of aged humans, nonhuman primates, canines, and rodents exhibit increased expression of MHC class II, complement receptors, TLR4, and CD14 [10, 13, 48, 50]. Subsequent to peripheral innate immune stimulation, microglial cells in aged brains respond with an exaggerated inflammatory response compared with younger cohorts [10, 11]. However, it should be noted that the precise priming stimulus of aging has yet to be identified.
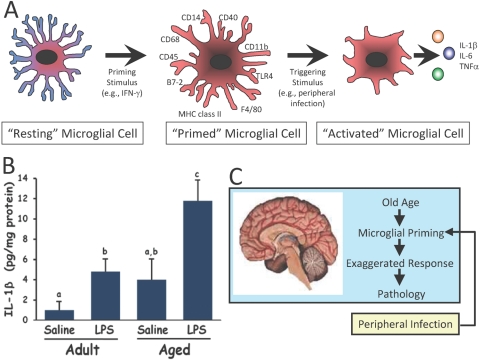
Fig. 2.
Microglial cells in the aged brain elicit a discordant inflammatory response upon activation by the peripheral innate immune system. (A) Similar to the priming paradigm proposed for peripheral macrophages, microglial cells in the aged brain may be primed as characterized by phenotypic alterations (e.g., increased expression of cell-surface markers). Upon receiving a triggering stimulus, these primed microglia release excessive concentrations of inflammatory cytokines in the CNS. (B) Evidence suggests the aged mouse brain responds to peripheral infection with a more exaggerated cytokine response compared with the adult mouse brain. Subsequent to peripheral immune stimulation with lipopolysaccharide (LPS), the release of IL-1β is higher in the aged vs. adult mouse brain [10]. (C) Both priming and triggering stimuli appear necessary to cause a discordant response by microglial cells in the aged brain. Cytokines released by microglia normally initiate an adaptive sickness behavior syndrome that includes anorexia, fever, and decreased social exploration. However, excessive cytokine release by primed and activated microglia elicits a discordant, maladaptive sickness behavior syndrome in the aged animal.
In the case of neurodegenerative diseases, abnormal proteins specific for each condition (e.g., β-amyloid) have long been implicated as microglial activating factors [45], so it may be speculated that low levels of these proteins may serve as microglial priming stimuli in the early stages of disease progression. It is also widely accepted that substances released from damaged CNS cells elicit microglial activation, and thus it may be possible that constant elimination of cellular debris over the lifetime of an organism may serve to prime microglial cells. Additionally, because injured neurons are capable of directly activating microglia, it is possible that aberrant control over this process occurs in the aged brain, ultimately leading to primed microglia. Whereas other microglial activating signals have been identified (e.g., ATP), it is difficult to speculate on the importance of such factors for microglial priming due to the (presumed) requisite chronic nature of such a stimulus. Interestingly, studies have suggested that IFN-γ concentrations are increased in the aged brain [51], which may implicate IFN-γ as a candidate molecule for microglial priming. To this end, the treatment of human microglial cell cultures with IFN-γ results in microglial activation, as evidenced by increased production of reactive oxygen species (i.e., superoxide) [52]. This scheme parallels the activation process of peripheral macrophages, wherein classical activation of macrophages requires IFN-γ prior to a secondary antigenic trigger [53]. Other studies indicate that aging is associated with a reduction in anti-inflammatory cytokines; for example, down-regulation of IL-4 signaling in the hippocampus of aged rats contributes to deficits in long-term potentiation [54]. Therefore, research focusing on the precise mechanism by which microglial cells are primed in the aged brain is certainly warranted, but regardless, currently available evidence supports the working hypothesis that progressive age is associated with priming of brain microglial cells.
Discordant central response to peripheral immune signals
Regardless of whether aged microglia are technically primed or merely sensitized, it is difficult to ignore the amplified central inflammatory response to peripheral infection that occurs in aged organisms. This central response is mediated primarily through the release of cytokines, especially IL-1β, IL-6, and TNF-α [55,56,57,58]. Ye and Johnson [59] showed that IL-6 was elevated not only in whole brain of aged mice, but also in discrete brain regions, including the hippocampus, cerebral cortex, and cerebellum, when compared with younger cohorts. Low-grade neuroinflammation has been suggested to be involved in cognitive aging and may facilitate neurodegenerative diseases [60]. In the case of Alzheimer’s disease, pathological changes in the suprachiasmatic nucleus disrupt circadian rhythms, which consequently induce cognitive abnormalities. Normal diurnal variations in cytokine patterns (including IL-1β), specifically in the hippocampus, hypothalamus, and cortical regions have long been implicated in modulating sleep patterns [61]. This point begs the question of whether discordant cytokine variations are natural and unavoidable events. Fluctuating cytokine concentrations have been implicated in the sundown syndrome associated with Alzheimer’s disease; sundowning is defined as the appearance or exacerbation of behavioral disturbances associated with the afternoon and/or evening hours [62, 63]. Thus, studies providing evidence of discordant or dysregulated cytokine patterns are useful in comprehending behavioral abnormalities associated with neurodegenerative diseases and possibly aging. Also interesting is the crucial role that cytokines play in altering sickness-associated behaviors. The release of cytokines in the brain during infection culminates in behavior modifications collectively known as the sickness behavior syndrome [44]; it includes behavioral outcomes such as anorexia, hypersomnia, lethargy, decreased social interaction, and deficits in cognitive and motor function [64, 65]. These behavioral changes are designed to protect the organism from further harm while the immune system mitigates peripheral infection; duration of disease can be lessened by succumbing to these behavioral modifications. Whereas sickness behavior is normally adaptive and reversible, the overproduction of cytokines in the aged brain may lead to pathological or maladaptive behavioral changes. Overall, assessment of these behavioral outcomes provides an objective and pertinent measure of immunological responses when characterizing age-related differences in experimental animals.
Using a murine model of aging, our laboratory has focused on the effects of i.p. LPS administration on sickness behavior in adult (4-6 months) vs. aged (22-24 months) animals. Interestingly, adult and aged mice show similar sickness behavior both in extent and timing of response (maximal response ∼2 h after LPS injection). However, adult mice begin recovering from LPS-induced effects after 4 h and are fully recovered by 24 h, whereas aged mice begin the recovery phase after 8 h and do not fully recover by 24 h [10, 11, 14]. These observations support the general consensus that immunological processes may not function properly with progressive age. To elucidate whether prolonged sickness behavior in aged animals resulted from an aberrant peripheral signal or increased CNS responsiveness, Huang et al. [13] administered intracerebroventricular LPS and observed that aged mice still exhibited an extended reduction in food intake, locomotor activity, and social exploration compared with younger mice. Thus, direct activation of the central innate immune system appears to occur even in the absence of peripheral signals, suggesting that regulation of periphery-to-brain communication cannot fully explain the discordant inflammatory response; these changes may be reflective of a persistent neuroinflammatory state in the aged brain. Importantly, we recently found IL-1ra given intracerebroventricularly to old mice reduced the depression in social behavior caused by peripheral LPS and facilitated recovery (unpublished data).
Brain regions including the hippocampus, cerebral cortex, and cerebellum are necessary for integration of functions, including learning and memory, spatial navigation, and motor coordination. Interestingly, a number of cytokine receptors have been characterized in these brain regions [66,67,68,69], which provides a direct link between discordant neuroinflammation and deficits in cognitive processing. As such, IL-1β has been shown to directly interrupt long-term potentiation [54, 70, 71], a neural process that seems to be an important substrate for learning and memory. Additional evidence of this point comes from studies investigating cytokine effects in animal behavioral models. Administration of IL-1β neutralizing antibodies mitigated the acute cognitive deficits caused by Legionella pneumophila infection in rodents tested in the Morris water maze test [72], while direct administration of IL-1β impaired spatial navigation learning in a similar behavioral task [73]. Therefore, cytokines appear to be important regulators of cognitive dysfunction associated with peripheral infection.
Not surprisingly, proinflammatory cytokine production is significantly greater in the hippocampus of aged mice compared with adult animals following treatment with a peripheral immune stimulus [11]. Additionally, LPS treatment induced deficits in spatial working memory in aged mice, while cognitive function was unaffected in young adult cohorts [11]. A similar finding was reported in an aged rat model, wherein Escherichia coli inoculation elicited an immune response and consequently impaired memory consolidation in a spatial memory task [12]. Additionally, recent evidence from our laboratory suggested that systemic absence of IL-6, a proinflammatory cytokine up-regulated during normal aging, prevented LPS-induced impairments of spatial working memory in mice [74]. The discordant inflammatory response and consequent depression in social exploration due to peripheral LPS administration in aged animals can also be mitigated through intracerebroventricular administration of IL-1ra (unpublished data). Therefore, strong evidence suggests that aberrant release of cytokines by primed microglial cells in the CNS is at least partly responsible for the exaggerated behavioral modifications observed in aged subjects exposed to peripheral infection. It is also likely that the importance of the aforementioned findings in rodent models will extend to elderly human subjects. A strong correlation exists between peripheral infection and disruptions in cognitive processing, as suggested by studies in humans and animals [12, 75,76,77,78]. Thus, a discordant central inflammatory response to peripheral infection in the aged brain may underlie acute cognitive impairments (i.e., delirium) commonly experienced by elderly patients [15]. Consequently, bouts of delirium increase the likelihood of poor self-care behaviors (i.e., anorexia, weight loss, noncompliance), and ultimately lead to increased rates of hospitalization and mortality [79, 80]. Therefore, the association between normal aging and exaggerated immunological responses in otherwise healthy individuals will remain an important area of research, especially given the rapid expansion of the segment of the human population aged 65 years and older.
CONCLUSION
Reduced immunological functioning in the elderly makes this population particularly susceptible to infection. Therefore, the concept that a discordant inflammatory response may result from fundamental differences in the sensitivity of brain microglia in old vs. young subjects is important. Controlling severity and duration of the behavioral response following a peripheral infection is paramount to improving health and quality of life of afflicted individuals. Thus, development of therapeutic strategies to counteract the effects of aging will only be possible after characterizing the basis for the aberrant regulation of centrally mediated neuroinflammation.
Acknowledgments
Supported by National Institutes of Health grants AG016710, AG023580, and MH069148.
References
- Dantzer R, O′Connor J C, Freund G G, Johnson R W, Kelley K W. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combrinck M I, Perry V H, Cunningham C. Peripheral infection evokes exaggerated sickness behaviour in pre-clinical murine prion disease. Neuroscience. 2002;112:7–11. doi: 10.1016/s0306-4522(02)00030-1. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Wilcockson D C, Campion S, Lunnon K, Perry V H. Central and systemic endotoxin challenges exacerbate the local inflammatory response and increase neuronal death during chronic neurodegeneration. J Neurosci. 2005;25:9275–9284. doi: 10.1523/JNEUROSCI.2614-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen M D, D′Aigle T, Gowing G, Julien J P, Rivest S. Exacerbation of motor neuron disease by chronic stimulation of innate immunity in a mouse model of amyotrophic lateral sclerosis. J Neurosci. 2004;24:1340–1349. doi: 10.1523/JNEUROSCI.4786-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry V H, Cunningham C, Holmes C. Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immunol. 2007;7:161–167. doi: 10.1038/nri2015. [DOI] [PubMed] [Google Scholar]
- Sibley W A, Bamford C R, Clark K. Clinical viral infections and multiple sclerosis. Lancet. 1985;1:1313–1315. doi: 10.1016/S0140-6736(85)92801-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmes C, El-Okl M, Williams A L, Cunningham C, Wilcockson D, Perry V H. Systemic infection, interleukin 1β, and cognitive decline in Alzheimer′s disease. J Neurol Neurosurg Psych. 2003;74:788–789. doi: 10.1136/jnnp.74.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry V H. The influence of systemic inflammation on inflammation in the brain: implications for chronic neurodegenerative disease. Brain Behav Immun. 2004;18:407–413. doi: 10.1016/j.bbi.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Johnson R W, Godbout J P. Aging, neuroinflammation, and behavior. Ader R, editor. Burlington, MA: Elsevier Academic Press; 2006:379–391. [Google Scholar]
- Godbout J P, Chen J, Abraham J, Richwine A F, Berg B M, Kelley K W, Johnson R W. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB J. 2005;19:1329–1331. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Chen J, Buchanan J B, Sparkman N L, Godbout J P, Freund G G, Johnson R W. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun. 2007;22:301–311. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos R M, Higgins E A, Biedenkapp J C, Sprunger D B, Wright-Hardesty K J, Watkins L R, Rudy J W, Maier S F. Peripheral infection and aging interact to impair hippocampal memory consolidation. Neurobiol Aging. 2006;27:723–732. doi: 10.1016/j.neurobiolaging.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Huang Y, Henry C J, Dantzer R, Johnson R W, Godbout J P. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiol Aging. 2007 doi: 10.1016/j.neurobiolaging.2007.04.012. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godbout J P, Moreau M, Lestage J, Chen J, Sparkman N L, Connor J O, Castanon N, Kelley K W, Dantzer R, Johnson R W. Aging Exacerbates Depressive-like Behavior in Mice in Response to Activation of the Peripheral Innate Immune System. Neuropsychopharmacology. 2007 doi: 10.1038/sj.npp.1301649. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wofford J L, Loehr L R, Schwartz E. Acute cognitive impairment in elderly ED patients: etiologies and outcomes. Am J Emerg Med. 1996;14:649–653. doi: 10.1016/S0735-6757(96)90080-7. [DOI] [PubMed] [Google Scholar]
- Chiovenda P, Vincentelli G M, Alegiani F. Cognitive impairment in elderly ED patients: need for multidimensional assessment for better management after discharge. Am J Emerg Med. 2002;20:332–335. doi: 10.1053/ajem.2002.33785. [DOI] [PubMed] [Google Scholar]
- Janssens J P, Krause K H. Pneumonia in the very old. Lancet Infect Dis. 2004;4:112–124. doi: 10.1016/S1473-3099(04)00931-4. [DOI] [PubMed] [Google Scholar]
- Janeway C A, Jr, Medzhitov R. Innate immune recognition. Annu Rev Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Blalock J E. The syntax of immune-neuroendocrine communication. Immunol Today. 1994;15:504–511. doi: 10.1016/0167-5699(94)90205-4. [DOI] [PubMed] [Google Scholar]
- Quan N, Banks W A. Brain-immune communication pathways. Brain Behav Immun. 2007;21:727–735. doi: 10.1016/j.bbi.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Breder C D, Dinarello C A, Saper C B. Interleukin-1 immunoreactive innervation of the human hypothalamus. Science. 1988;240:321–324. doi: 10.1126/science.3258444. [DOI] [PubMed] [Google Scholar]
- Komaki G, Arimura A, Koves K. Effect of intravenous injection of IL-1 beta on PGE2 levels in several brain areas as determined by microdialysis. Am J Physiol. 1992;262:E246–E251. doi: 10.1152/ajpendo.1992.262.2.E246. [DOI] [PubMed] [Google Scholar]
- Banks W A, Kastin A J. Blood to brain transport of interleukin links the immune and central nervous systems. Life Sci. 1991;48:PL117–PL121. doi: 10.1016/0024-3205(91)90385-o. [DOI] [PubMed] [Google Scholar]
- Gutierrez E G, Banks W A, Kastin A J. Murine tumor necrosis factor alpha is transported from blood to brain in the mouse. J Neuroimmunol. 1993;47:169–176. doi: 10.1016/0165-5728(93)90027-v. [DOI] [PubMed] [Google Scholar]
- Banks W A, Kastin A J, Broadwell R D. Passage of cytokines across the blood-brain barrier. Neuroimmunomodulation. 1995;2:241–248. doi: 10.1159/000097202. [DOI] [PubMed] [Google Scholar]
- Fabry Z, Fitzsimmons K M, Herlein J A, Moninger T O, Dobbs M B, Hart M N. Production of the cytokines interleukin 1 and 6 by murine brain microvessel endothelium and smooth muscle pericytes. J Neuroimmunol. 1993;47:23–34. doi: 10.1016/0165-5728(93)90281-3. [DOI] [PubMed] [Google Scholar]
- Ching S, Zhang H, Belevych N, He L, Lai W, Pu X A, Jaeger L B, Chen Q, Quan N. Endothelial-specific knockdown of interleukin-1 (IL-1) type 1 receptor differentially alters CNS responses to IL-1 depending on its route of administration. J Neurosci. 2007;27:10476–10486. doi: 10.1523/JNEUROSCI.3357-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dantzer R, Bluthe R M, Laye S, Bret-Dibat J L, Parnet P, Kelley K W. Cytokines and sickness behavior. Ann N Y Acad Sci. 1998;840:586–590. doi: 10.1111/j.1749-6632.1998.tb09597.x. [DOI] [PubMed] [Google Scholar]
- Watkins L R, Wiertelak E P, Goehler L E, Mooney-Heiberger K, Martinez J, Furness L, Smith K P, Maier S F. Neurocircuitry of illness-induced hyperalgesia. Brain Res. 1994;639:283–299. doi: 10.1016/0006-8993(94)91742-6. [DOI] [PubMed] [Google Scholar]
- Bluthe R M, Walter V, Parnet P, Laye S, Lestage J, Verrier D, Poole S, Stenning B E, Kelley K W, Dantzer R. Lipopolysaccharide induces sickness behaviour in rats by a vagal mediated mechanism. C R Acad Sci III. 1994;317:499–503. [PubMed] [Google Scholar]
- Goehler L E, Relton J K, Dripps D, Kiechle R, Tartaglia N, Maier S F, Watkins L R. Vagal paraganglia bind biotinylated interleukin-1 receptor antagonist: a possible mechanism for immune-to-brain communication. Brain Res Bull. 1997;43:357–364. doi: 10.1016/s0361-9230(97)00020-8. [DOI] [PubMed] [Google Scholar]
- Goehler L E, Gaykema R P, Nguyen K T, Lee J E, Tilders F J, Maier S F, Watkins L R. Interleukin-1β in immune cells of the abdominal vagus nerve: a link between the immune and nervous systems? J Neurosci. 1999;19:2799–2806. doi: 10.1523/JNEUROSCI.19-07-02799.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalton D K, Pitts-Meek S, Keshav S, Figari I S, Bradley A, Stewart T A. Multiple defects of immune cell function in mice with disrupted interferon-γ genes. Science. 1993;259:1739–1742. doi: 10.1126/science.8456300. [DOI] [PubMed] [Google Scholar]
- Gordon S. Alternative activation of macrophages. Nat Rev Immunol. 2003;3:23–35. doi: 10.1038/nri978. [DOI] [PubMed] [Google Scholar]
- Schroder K, Sweet M J, Hume D A. Signal integration between IFN-γ and TLR signalling pathways in macrophages. Immunobiology. 2006;211:511–524. doi: 10.1016/j.imbio.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Perry V H, Newman T A, Cunningham C. The impact of systemic infection on the progression of neurodegenerative disease. Nat Rev Neurosci. 2003;4:103–112. doi: 10.1038/nrn1032. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Deacon R, Wells H, Boche D, Waters S, Diniz C P, Scott H, Rawlins J N, Perry V H. Synaptic changes characterize early behavioural signs in the ME7 model of murine prion disease. Eur J Neurosci. 2003;17:2147–2155. doi: 10.1046/j.1460-9568.2003.02662.x. [DOI] [PubMed] [Google Scholar]
- Somera-Molina K C, Robin B, Somera C A, Anderson C, Stine C, Koh S, Behanna H A, Van Eldik L J, Watterson D M, Wainwright M S. Glial activation links early-life seizures and long-term neurologic dysfunction: evidence using a small molecule inhibitor of proinflammatory cytokine upregulation. Epilepsia. 2007;48:1785–1800. doi: 10.1111/j.1528-1167.2007.01135.x. [DOI] [PubMed] [Google Scholar]
- Stichel C C, Luebbert H. Inflammatory processes in the aging mouse brain: participation of dendritic cells and T-cells. Neurobiol Aging. 2007;28:1507–1521. doi: 10.1016/j.neurobiolaging.2006.07.022. [DOI] [PubMed] [Google Scholar]
- Galea I, Bernardes-Silva M, Forse P A, van Rooijen N, Liblau R S, Perry V H. An antigen-specific pathway for CD8 T cells across the blood-brain barrier. J Exp Med. 2007;204:2023–2030. doi: 10.1084/jem.20070064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloss C U, Bohatschek M, Kreutzberg G W, Raivich G. Effect of lipopolysaccharide on the morphology and integrin immunoreactivity of ramified microglia in the mouse brain and in cell culture. Exp Neurol. 2001;168:32–46. doi: 10.1006/exnr.2000.7575. [DOI] [PubMed] [Google Scholar]
- Buttini M, Limonta S, Boddeke H W. Peripheral administration of lipopolysaccharide induces activation of microglial cells in rat brain. Neurochem Int. 1996;29:25–35. doi: 10.1016/0197-0186(95)00141-7. [DOI] [PubMed] [Google Scholar]
- Kelley K W, Bluthe R M, Dantzer R, Zhou J H, Shen W H, Johnson R W, Broussard S R. Cytokine-induced sickness behavior. Brain Behav Immun. 2003;17:S112–S118. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Garden G A, Moller T. Microglia biology in health and disease. J Neuroimmune Pharmacol. 2006;1:127–137. doi: 10.1007/s11481-006-9015-5. [DOI] [PubMed] [Google Scholar]
- Streit W J. Microglial senescence: does the brain′s immune system have an expiration date? Trends Neurosci. 2006;29:506–510. doi: 10.1016/j.tins.2006.07.001. [DOI] [PubMed] [Google Scholar]
- Frank M G, Barrientos R M, Biedenkapp J C, Rudy J W, Watkins L R, Maier S F. mRNA up-regulation of MHC II and pivotal pro-inflammatory genes in normal brain aging. Neurobiol Aging. 2006;27:717–722. doi: 10.1016/j.neurobiolaging.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Perry V H, Matyszak M K, Fearn S. Altered antigen expression of microglia in the aged rodent CNS. Glia. 1993;7:60–67. doi: 10.1002/glia.440070111. [DOI] [PubMed] [Google Scholar]
- Sheffield L G, Berman N E. Microglial expression of MHC class II increases in normal aging of nonhuman primates. Neurobiol Aging. 1998;19:47–55. doi: 10.1016/s0197-4580(97)00168-1. [DOI] [PubMed] [Google Scholar]
- Letiembre M, Hao W, Liu Y, Walter S, Mihaljevic I, Rivest S, Hartmann T, Fassbender K. Innate immune receptor expression in normal brain aging. Neuroscience. 2007;146:248–254. doi: 10.1016/j.neuroscience.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Maher F O, Clarke R M, Kelly A, Nally R E, Lynch M A. Interaction between interferon gamma and insulin-like growth factor-1 in hippocampus impacts on the ability of rats to sustain long-term potentiation. J Neurochem. 2006;96:1560–1571. doi: 10.1111/j.1471-4159.2006.03664.x. [DOI] [PubMed] [Google Scholar]
- Chao C C, Hu S, Peterson P K. Modulation of human microglial cell superoxide production by cytokines. J Leukoc Biol. 1995;58:65–70. doi: 10.1002/jlb.58.1.65. [DOI] [PubMed] [Google Scholar]
- Davila D R, Edwards C K, III, Arkins S, Simon J, Kelley K W. Interferon-gamma-induced priming for secretion of superoxide anion and tumor necrosis factor-alpha declines in macrophages from aged rats. FASEB J. 1990;4:2906–2911. doi: 10.1096/fasebj.4.11.2165948. [DOI] [PubMed] [Google Scholar]
- Maher F O, Nolan Y, Lynch M A. Downregulation of IL-4-induced signalling in hippocampus contributes to deficits in LTP in the aged rat. Neurobiol Aging. 2005;26:717–728. doi: 10.1016/j.neurobiolaging.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Hager K, Machein U, Krieger S, Platt D, Seefried G, Bauer J. Interleukin-6 and selected plasma proteins in healthy persons of different ages. Neurobiol Aging. 1994;15:771–772. doi: 10.1016/0197-4580(94)90066-3. [DOI] [PubMed] [Google Scholar]
- Wei J, Xu H, Davies J L, Hemmings G P. Increase of plasma IL-6 concentration with age in healthy subjects. Life Sci. 1992;51:1953–1956. doi: 10.1016/0024-3205(92)90112-3. [DOI] [PubMed] [Google Scholar]
- Daynes R A, Araneo B A, Ershler W B, Maloney C, Li G Z, Ryu S Y. Altered regulation of IL-6 production with normal aging. Possible linkage to the age-associated decline in dehydroepiandrosterone and its sulfated derivative. J Immunol. 1993;150:5219–5230. [PubMed] [Google Scholar]
- Ershler W B. Interleukin-6: a cytokine for gerontologists. J Am Geriatr Soc. 1993;41:176–181. doi: 10.1111/j.1532-5415.1993.tb02054.x. [DOI] [PubMed] [Google Scholar]
- Ye S M, Johnson R W. Increased interleukin-6 expression by microglia from brain of aged mice. J Neuroimmunol. 1999;93:139–148. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- Wilson C J, Finch C E, Cohen H J. Cytokines and cognition–the case for a head-to-toe inflammatory paradigm. J Am Geriatr Soc. 2002;50:2041–2056. doi: 10.1046/j.1532-5415.2002.50619.x. [DOI] [PubMed] [Google Scholar]
- Taishi P, Bredow S, Guha-Thakurta N, Obal F, Jr, Krueger J M. Diurnal variations of interleukin-1 beta mRNA and beta-actin mRNA in rat brain. J Neuroimmunol. 1997;75:69–74. doi: 10.1016/s0165-5728(97)00002-7. [DOI] [PubMed] [Google Scholar]
- Volicer L, Harper D G, Manning B C, Goldstein R, Satlin A. Sundowning and circadian rhythms in Alzheimer′s disease. Am J Psychiatry. 2001;158:704–711. doi: 10.1176/appi.ajp.158.5.704. [DOI] [PubMed] [Google Scholar]
- Little J T, Satlin A, Sunderland T, Volicer L. Sundown syndrome in severely demented patients with probable Alzheimer′s disease. J Geriatr Psychiatry Neurol. 1995;8:103–106. doi: 10.1177/089198879500800205. [DOI] [PubMed] [Google Scholar]
- Konsman J P, Parnet P, Dantzer R. Cytokine-induced sickness behaviour: mechanisms and implications. Trends Neurosci. 2002;25:154–159. doi: 10.1016/s0166-2236(00)02088-9. [DOI] [PubMed] [Google Scholar]
- Pollmacher T, Haack M, Schuld A, Reichenberg A, Yirmiya R. Low levels of circulating inflammatory cytokines—do they affect human brain functions? Brain Behav Immun. 2002;16:525–532. doi: 10.1016/s0889-1591(02)00004-1. [DOI] [PubMed] [Google Scholar]
- Parnet P, Kelley K W, Bluthe R M, Dantzer R. Expression and regulation of interleukin-1 receptors in the brain. Role in cytokines-induced sickness behavior. J Neuroimmunol. 2002;125:5–14. doi: 10.1016/s0165-5728(02)00022-x. [DOI] [PubMed] [Google Scholar]
- Loddick S A, Liu C, Takao T, Hashimoto K, De Souza E B. Interleukin-1 receptors: cloning studies and role in central nervous system disorders. Brain Res Rev. 1998;26:306–319. doi: 10.1016/s0165-0173(97)00037-4. [DOI] [PubMed] [Google Scholar]
- Gadient R A, Otten U. Identification of interleukin-6 (IL-6)-expressing neurons in the cerebellum and hippocampus of normal adult rats. Neurosci Lett. 1994;182:243–246. doi: 10.1016/0304-3940(94)90807-9. [DOI] [PubMed] [Google Scholar]
- Takao T, Culp S G, Newton R C, De Souza E B. Type I interleukin-1 receptors in the mouse brain-endocrine-immune axis labelled with [125I]recombinant human interleukin-1 receptor antagonist. J Neuroimmunol. 1992;41:51–60. doi: 10.1016/0165-5728(92)90195-q. [DOI] [PubMed] [Google Scholar]
- Griffin R, Nally R, Nolan Y, McCartney Y, Linden J, Lynch M A. The age-related attenuation in long-term potentiation is associated with microglial activation. J Neurochem. 2006;99:1263–1272. doi: 10.1111/j.1471-4159.2006.04165.x. [DOI] [PubMed] [Google Scholar]
- Murray C A, Lynch M A. Evidence that increased hippocampal expression of the cytokine interleukin-1β is a common trigger for age- and stress-induced impairments in long-term potentiation. J Neurosci. 1998;18:2974–2981. doi: 10.1523/JNEUROSCI.18-08-02974.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibertini M. IL1β impairs relational but not procedural rodent learning in a water maze task. Adv Exp Med Biol. 1996;402:207–217. doi: 10.1007/978-1-4613-0407-4_27. [DOI] [PubMed] [Google Scholar]
- Oitzl M S, van Oers H, Schobitz B, de Kloet E R. Interleukin-1β, but not interleukin-6, impairs spatial navigation learning. Brain Res. 1993;613:160–163. doi: 10.1016/0006-8993(93)90468-3. [DOI] [PubMed] [Google Scholar]
- Sparkman N L, Buchanan J B, Heyen J R, Chen J, Beverly J L, Johnson R W. Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci. 2006;26:10709–10716. doi: 10.1523/JNEUROSCI.3376-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibertini M. Cytokines and cognitive behavior. Neuroimmunomodulation. 1998;5:160–165. doi: 10.1159/000026332. [DOI] [PubMed] [Google Scholar]
- Pugh C R, Kumagawa K, Fleshner M, Watkins L R, Maier S F, Rudy J W. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behav Immun. 1998;12:212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- Arai K, Matsuki N, Ikegaya Y, Nishiyama N. Deterioration of spatial learning performances in lipopolysaccharide-treated mice. Jpn J Pharmacol. 2001;87:195–201. doi: 10.1254/jjp.87.195. [DOI] [PubMed] [Google Scholar]
- Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- Armstrong G L, Conn L A, Pinner R W. Trends in infectious disease mortality in the United States during the 20th century. JAMA. 1999;281:61–66. doi: 10.1001/jama.281.1.61. [DOI] [PubMed] [Google Scholar]
- Johnston M, Wakeling A, Graham N, Stokes F. Cognitive impairment, emotional disorder and length of stay of elderly patients in a district general hospital. Br J Med Psychol. 1987;60:133–139. doi: 10.1111/j.2044-8341.1987.tb02723.x. [DOI] [PubMed] [Google Scholar]