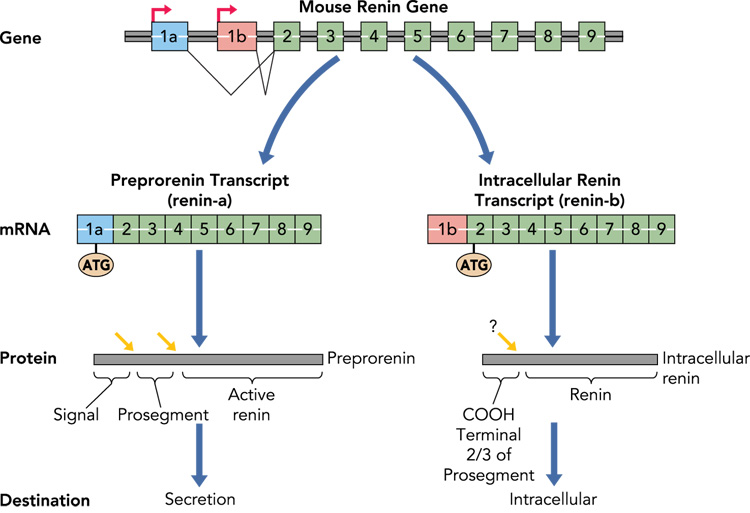
Figure 2. Synthesis of Intracellular Renin.
The hypothesized biosynthetic pathway for intracellular renin is shown. Classically, preprorenin is the primary translation product of the renin-A mRNA transcribed from the classical renin promoter and including exon-1a. Preprorenin is processed (red arrows) first by removal of the signal peptide and then by removal of the prosegment. Active renin is subsequently released into the systemic circulation. In the brain, a different renin mRNA termed renin-b is transcribed from an unknown promoter within intron-1 (in mice) or 6.2 kb upstream of the classical renin promoter (in humans) to result in a novel transcript lacking exon-1a, and including exon-1b. There are no ATG sequences in exon-1b in rat, mouse, or human, and thus translation begins at the highly conserved ATG present in exon-2. This product encodes the entire active renin protein and ⅔ of the prosegment. It is unclear, if the prosegment is removed. As the protein lacks a signal peptide, it is unlikely to be secreted. Additional studies are needed to determine if this protein is stable, remains intracellularly, and within which intracellular structures it resides.