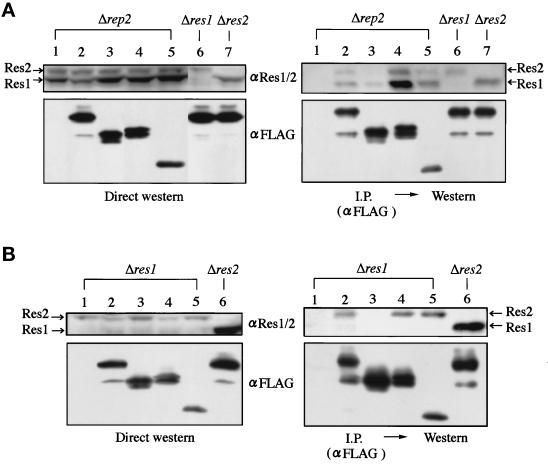
Figure 4.
The putative Res2 binding domain in Rep2 is required for its Res2 binding in vivo. Full-length and deletion mutants of rep2+ tagged with FLAG epitope were inserted into the pREP1 vector and transfected into the rep2 (N3–141s), res1 (K156-D1), and res2 disruptants (M222). The structures of rep2+ deletion mutants are shown in Figure 3A except for the Δ22–131 aa mutant, which was constructed by fusing the sequence of amino acids 1–21 with NΔ131. Cell extracts (4.5 mg protein) prepared from the transformants grown in thiamine-free medium at 30°C to express the FLAG-tagged Rep2 were immnoprecipitated with the anti-FLAG antibody. Cell extracts (200 μg protein) (left panels) and immunoprecipitates (right panels) were separated in SDS-polyacrylamide gels and analyzed by Western blotting with anti-Res1/2 or anti-FLAG antibody. Lane 1, empty vector; lanes 2, 6, 7, full-length rep2+; lane 3, Δ132–176 aa; lane 4, CΔ43; lane 5, Δ22–131 aa.