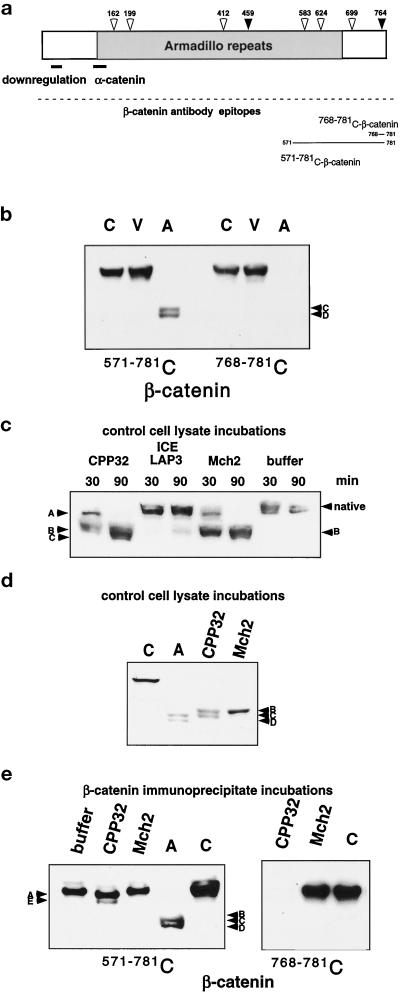
Figure 2.
Possible involvement of the caspases in the cleavage of β-catenin in HUVEC apoptosis. (a) A schematic diagram of β-catenin shows possible caspase cleavage sites: CPP32-like sites (black arrows) and Mch2-like sites (white arrows) indicating consensus cleavage sites with amino acid sequences of DXXD and (L/I/V)XXD, respectively. The α-catenin–binding site and regions important for down-regulation of β-catenin are illustrated with a line, and the region of armadillo repeats is indicated in gray. The epitopes recognized by the antibodies used for analysis are also shown. (b) Control (C), viable (V), and apoptotic (A) cell lysates were analyzed by SDS-PAGE and Western blotting with either a monoclonal antibody (571–781 C) or a polyclonal antibody (768–781 C) to the indicated C-terminal peptides of β-catenin. The polyclonal antibody to amino acids 768–781 of β-catenin does not recognize the apoptotic fragments C and D, which indicates that the C terminus of the molecule is lost. (c) Lysates from control HUVEC were incubated with recombinant caspases CPP32, ICE LAP3, and Mch2 or reaction buffer for 30 and 90 min, subjected to 7.5% SDS-PAGE, and analyzed by Western blotting with antibody 768–781 C-β-catenin. After just 30 min incubation with CPP32, no native β-catenin remains, and fragments A and B (comparable to those observed in the time course of endothelial apoptosis, Figure 1b) are prominent. After 90 min, fragment A is no longer detectable and fragment C becomes apparent; while even after a 90-min incubation with ICE LAP3, no significant cleavage is observed; primarily, fragment B is observed after Mch2 incubation. (d) Lysates from control HUVEC were incubated with recombinant CPP32 and Mch2 for 45 min, subjected to 7.5% SDS-PAGE, and analyzed by Western blotting with antibody 571–781 C-β-catenin. Lysates from control (C) and apoptotic cells (A) were included for comparison. In control lysates, β-catenin is cleaved by both CPP32 and Mch2, but the final in vitro cleavage products (fragments B and C with CPP32 and only fragment B with Mch2) differ from fragments of β-catenin in apoptotic HUVEC (fragments C and D). (e) Immunoprecipitated β-catenin was incubated with recombinant CPP32, recombinant Mch2, or no enzyme (buffer), and compared with control (C) and apoptotic (A) cell lysates after SDS-PAGE and Western analysis with antibodies 768–781 C or 571–781 C to the indicated C-terminal peptides of β-catenin. Antibody 768–781 C does not recognize immunoprecipitated β-catenin that was cleaved by CPP32. This finding and the minor band shift from cleavage suggest that CPP32 cleaves at the most proximal DXXD located at the C terminus (764 in panel a).