Abstract
Mammalian sleep is not a homogenous state, and different variables have traditionally been used to distinguish different periods during sleep. Of these variables, eye movement is one of the most paradigmatic, and has been used to differentiate between the so-called rapid eye movement (REM) and non-REM (NREM) sleep periods. Despite this, eye movements during sleep are poorly understood, and the behaviour of the oculomotor system remains almost unknown. In the present work, we recorded binocular eye movements during the sleep–wake cycle of adult cats by the scleral search-coil technique. During alertness, eye movements consisted of conjugated saccades and eye fixations. During NREM sleep, eye movements were slow and mostly unconjugated. The two eyes moved upwardly and in the abducting direction, producing a tonic divergence and elevation of the visual axis. During the transition period between NREM and REM sleep, rapid monocular eye movements of low amplitude in the abducting direction occurred in coincidence with ponto-geniculo-occipital waves. Along REM sleep, the eyes tended to maintain a tonic convergence and depression, broken by high-frequency bursts of complex rapid eye movements. In the horizontal plane, each eye movement in the burst comprised two consecutive movements in opposite directions, which were more evident in the eye that performed the abducting movements. In the vertical plane, rapid eye movements were always upward. Comparisons of the characteristics of eye movements during the sleep–wake cycle reveal the uniqueness of eye movements during sleep, and the noteworthy existence of tonic and phasic phenomena in the oculomotor system, not observed until now.
More than 50 years ago, Aserinsky & Kleitman (1953) reported the existence of periods of fast and jerky eye movements during sleep. Since then, sleep has been considered a non-homogeneous state, subdivided into rapid eye movement (REM) and non-REM (NREM) sleep. NREM sleep is mainly characterized by synchronized cortical activity that results from the connectivity between cortical and thalamic neurons and the intrinsic properties of neurons in each of these structures (Steriade, 2006). Besides eye movements, REM sleep is characterized by the absence of antigravity muscular tonus (Jouvet & Michel, 1959) with sporadic twitches (Chase & Morales, 1983) and high-frequency and low-amplitude electroencephalographic activity (Dement, 1958), and the presence of high-amplitude spiky potentials that are mainly recorded in pontine, geniculate and occipital areas, and named ponto-geniculo-occipital (PGO) waves (Jeannerod et al. 1965). Since the discovery of these cardinal signs, considerable effort has been made to understand the mechanisms underlying each of them (Steriade & McCarley, 2005).
In addition to identifying sleep phases, eye movements have been considered important because of their possible relationship with the scanning of visual images during dreaming (Roffwarg et al. 1962; Herman et al. 1984). However, the behaviour of the oculomotor system during sleep remains poorly understood. Although numerous studies in sleep research have attempted to characterize eye movements during REM sleep (Gottesmann, 1996), most of them used electrooculography (EOG), a technique with low resolution that does not allow knowing the true position of the eye in the orbit. In fact, there are only a few papers that – by using the scleral search-coil technique – accurately describe eye movements in monkeys (Fuchs & Ron, 1968; Zhou & King, 1997), cats (Vanni-Mercier et al. 1994) and guinea pigs (Escudero & Vidal, 1996). Nevertheless, each of these works is in some aspects incomplete. All of them except Zhou & King (1997) employed monocular recording, and only Vanni-Mercier et al. (1994) studied the relationship between eye movements and PGO waves. These works describe the existence of isolated and grouped rapid eye movements that induce looped trajectories during REM sleep. Rapid eye movements coincide with the occurrence of PGO waves at the lateral geniculate nuclei (LGN) (Vanni-Mercier et al. 1994) and are mostly unconjugated, while the visual axes of the eyes show some misalignments during REM sleep (Zhou & King, 1997). Dynamic properties of rapid eye movements during sleep are controversial. It has been reported that during REM sleep, the velocity of rapid eye movements in monkeys is lower than (Fuchs & Ron, 1968) or equal to (Zhou & King, 1997) that during alertness, whereas in cats (Vanni-Mercier et al. 1994) and guinea pigs (Escudero & Vidal, 1996) it is higher.
These differences between eye movements during alertness and sleep are important, not only because of their possible relation with the scanning of visual images, but because the oculomotor system could help us understand the behaviour of other motor systems during sleep. The oculomotor system is perhaps one of the most-studied motor systems in alert animals, and could constitute an excellent model in which to study functional implications of sleep.
Each eye is moved by the action of six extraocular muscles. The medial and lateral recti are involved exclusively in horizontal eye movements, whereas the superior and inferior recti and the superior and inferior oblique participate in the control of vertical and torsional eye movements. Abducting and adducting eye movements are controlled by motoneurons located in the abducens (ABD) and oculomotor nuclei, respectively. Internuclear interneurons in the ABD nucleus project to the contralateral medial rectus motoneurons, and are responsible for conjugated eye movements (Highstein et al. 1982). In the vertical plane, upward and downward movements are controlled by motoneurons located in the oculomotor and trochlear nuclei.
In this work, we have recorded and analysed the movement of both eyes along the sleep–wake cycle. The results show the existence of tonic and phasic phenomena whose temporal course and detailed characteristics have not been previously described.
Methods
Subjects
Nine adult female cats (2.4–3.7 kg) of European strains, obtained from an authorized supplier (University of Córdoba, Spain), were used as experimental subjects. Experiments were performed in accordance with the European Union directive 609/86/EU, the current Spanish legislation (RD 1201/2005), and the Animal Experimentation Ethics Committee of the University of Seville for the use of laboratory animals. Every effort was made to minimize the number of animals used and their suffering.
Chronic preparation
Animals were prepared for chronic recording of eye movements and for simultaneous recording of electroencephalography (EEG), electromyography of the trapezius (EMG), and PGO waves. Briefly, animals were anaesthetized with sodium pentobarbitone (35 mg kg−1, i.p.) following a protective injection of atropine (0.5 mg kg−1, i.m.) aimed at preventing vagal reflexes. Under aseptic conditions, the cats were implanted bilaterally with Teflon-coated stainless-steel coils (Cooner Wire, Chatsworth, CA, USA) sutured to the scleral margin of the eye. Screw electrodes and bipolar stainless-steel wire electrodes (A-M Systems, Everett, WA, USA) were implanted bilaterally over the frontal cortex and the trapezius neck muscles for EEG and EMG recording, respectively. Bipolar electrodes (A-M Systems) were implanted in the LGN for the recording of PGO waves during REM sleep. A head-holding system, consisting of three bolts cemented to the skull perpendicular to the stereotaxic plane, was also implanted. Eye coils and polysomnographic recording electrodes were connected to a socket attached to the holding system. The animal received post-operative systemic treatment with antibiotics, and anti-inflammatory and analgesic drugs. During the complete experimental period, antibiotics, local anaesthetics and corticoids were topically applied to the eyes.
Recording sessions
One to two weeks later, when there was a total recovery from surgery, animals were habituated to the experimental set-up. Every 2–4 days, for 2–3 h per day, animals were lightly restrained by elastic bandages, with their head fixed to the recording table by means of the head-holding system. The head of the cat was fixed 21 deg nose-down to maintain the horizontal semicircular canal in the horizontal plane. After one to five sessions, animals remained relaxed – their heart and respiratory rates were not different from those of free animals, showing no signs of discomfort or stress. Recording sessions were carried out for a maximum of 8–12 weeks. Spontaneous eye movements and polysomnographic activity were recorded continuously during the sleep–wake cycle. Eye movements were recorded with the scleral search-coil technique (CNC-Engineering, Seattle, CA, USA) (Fuchs & Robinson, 1966), and were calibrated at the beginning of each experimental session by rotating (± 10 deg) the magnetic field frame about both the horizontal and vertical planes. Eye position, EEG, EMG and PGO waves were stored using an eight-channel videotape recording system (Neurocorder, Neurodata Instruments, NY, USA), and fed into a computer for off-line analysis (Power 1401, Cambridge Instruments, Cambridge, UK). Eye-position signals and polysomnographic activity were sampled at 500 Hz and 5 kHz, respectively. At the end of the experimental period, animals were anaesthetized with sodium pentobarbitone (50 mg kg−1i.p.), and transcardially perfused with saline and 4% paraformaldehyde.
Analysis of eye movements
The ocular disparity between the two eyes along the sleep–wake cycle was calculated as the difference in their relative position. Upward and leftward rotations of the eyes were considered positive, and downward and rightward movements were negative. Subtraction of the right eye position from the left eye position in the horizontal plane denoted positive values for divergence and negative values for convergence. Similarly, interocular disparity was calculated for the vertical plane, with positive values indicating that the left eye was more upwardly rotated than the right eye, and vice versa. To obtain a representative value for interocular disparity during a time period, interocular disparity values were averaged along this time.
Possible differences in the codification of rapid eye movements during the sleep–wake cycle were examined by analysing the relationship between eye velocity and amplitude. Eye velocity was calculated as an average difference with an interval of 6 ms (Spike2, Cambridge Instruments). The peak velocity of the eye during the movement was plotted as a function of the amplitude, and a linear regression analysis was performed. The slope of the fitted line was used to compare the codification of rapid eye movements during sleep and alertness. Student's t test and one-way ANOVA for repeated measures were performed in SPSS (v 14.0, SPSS Inc.). Statistical significance was set at P < 0.05. All results are shown as mean ± s.d.
Results
The behaviour of the oculomotor system was studied during 69 sleep–wake cycles in nine animals. The position of both eyes, and EEG, trapezius EMG and PGO activities were recorded continuously along recording sessions. After eye movement calibrations and recording of spontaneous eye movements during alertness, animals were left in repose. As a rule, after a varying period of alertness, cats became drowsy, and sometime displayed a period of NREM sleep followed by REM sleep. Very frequently, an NREM sleep was followed by a period of alertness, but after 3–4 cycles of NREM alertness, there was a REM sleep. The mean duration of NREM and REM sleep was 327.9 ± 99.9 s and 398.1 ± 68.6 s (n = 10), respectively.
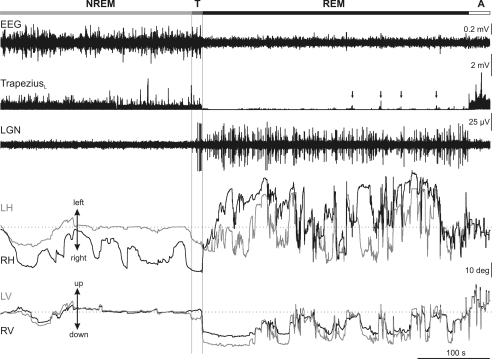
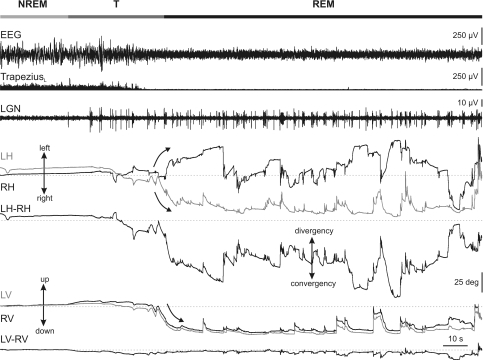
Figure 1 displays an example of EEG, left trapezius EMG and PGO wave activities, and horizontal and vertical eye movements, during a representative short sleep period. Polysomnographic recording enabled establishing the limits between NREM and REM sleep states. Thus, NREM sleep was identified by a high-amplitude synchronized EEG activity without PGO waves and by the presence of neck muscular tone, and REM sleep by a low-amplitude EEG recording, PGO wave activities, absence of muscular tonus, and muscular twitches. Between NREM and REM sleep, there was an intermediate phase, denoted hereafter ‘transition period’. Transitions from NREM to REM sleep were short periods of time in which small rapid eye movements, and isolated PGO waves of high amplitude at the LGN appeared, whereas the EEG still displayed high amplitude, and the neck muscle tonic activities typical of NREM sleep. We considered that transition periods finished when the first group of PGO waves appeared.
Figure 1. Eye movements during the sleep–wake cycle in the cat.
Representative recording of the electroencephalographic (EEG) activity, rectified electromyographic activity of the left trapezius, and lateral geniculate nucleus (LGN) activity, together with left (grey traces) and right (black traces) horizontal (LH, RH) and vertical (LV, RV) eye position along a short sleep–wake cycle. Polysomnographic recording enabled the distinguishing of four different periods in the sleep–wake cycle (horizontal bar at the top): non-rapid eye movement sleep (NREM), transition period (T), rapid eye movement sleep (REM), and awake (A). The transition period between NREM and REM sleep was characterized by the occurrence of high-amplitude ponto-geniculo-occipital waves at the LGN, with low frequencies in the EEG and tonic muscular activity. Note the different oculomotor behaviour during each period. During NREM sleep, the eyes performed slow movements that were generally not conjugated during this period. During REM sleep, the eyes performed bursts of movements of high amplitude and velocity that were greater in the horizontal plane. A tendency to maintain eccentric eye position in the horizontal and vertical planes was characteristic during REM sleep. Dotted lines in eye-position traces indicate the centre of the orbit. Absence of tonic signals and muscular twitches, indicated by vertical arrows in electromyographic recording, were observed during REM sleep. Calibrations are indicated at the right side of the figure.
Along sleep, binocular recording of eye position characteristically showed two types of phenomenon that affected the oculomotor system differentially: tonic phenomena, modulating the alignment of visual axes along each sleep phase, and phasic phenomena, in which rapid eye movements were associated with the occurrence of PGO waves at the LGN during the transition period and REM sleep.
Tonic phenomena affecting the oculomotor system along the sleep–wake cycle
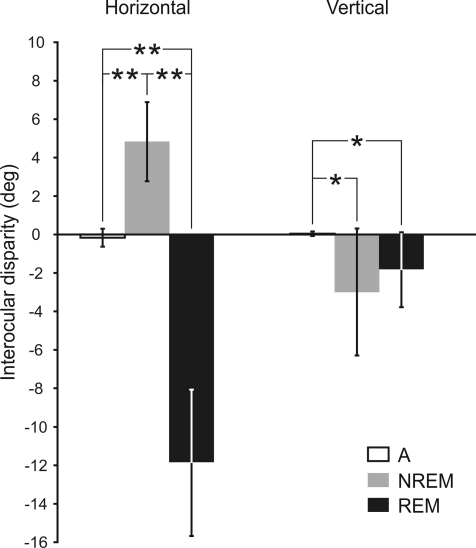
Alertness periods were characterized by low-amplitude EEG, muscular tonus at the neck, and absence of PGO waves at the LGN. Spontaneous eye movements during alertness consisted of rapid eye movements (saccades) that moved both eyes from one visual target to another, separated by periods of eye immobility that kept the gaze fixed on the targets (Fig. 2). These eye movements were conjugated, and the interocular disparity, calculated as the difference in position between the two eyes for the horizontal (LH–RH in Fig. 2) and vertical (LV–RV in Fig. 2) planes, was small. During alertness, the mean interocular disparity in the horizontal and vertical planes was −0.2 ± 0.5 deg (n = 9) and 0.0 ± 0.1 deg (n = 9) (Fig. 5), respectively.
Figure 2. Eye movements and interocular disparities during alertness.
Representative recording of the electroencephalographic (EEG) activity, rectified electromyographic activity of the left trapezius, and lateral geniculate nucleus (LGN) activity, together with left (grey traces) and right (black traces) horizontal (LH, RH) and vertical (LV, RV) eye position during alertness. This period was characterized by low-amplitude EEG activity, muscular tone and absence of ponto-geniculo-occipital waves at the LGN. Spontaneous eye movements during alertness consisted of rapid eye movements (saccades) that moved the eyes from one visual target to another, separated by periods of eye immobility that kept the gaze fixed on the target. Interocular disparity, calculated as the difference in position between the left and the right eye for the horizontal (LH–RH) and vertical (LV–RV) planes, was small. Dotted lines indicate the zero value for eye positions and interocular disparities.
Figure 5. Interocular disparity along sleep–wake cycle.
Interocular disparities in the horizontal and vertical planes were averaged along alertness (A, white bar), NREM sleep (grey bar), and REM sleep (black bar). In the horizontal plane, mean disparities during all periods were statistically significant (P < 0.01, **). In the vertical plane, mean disparities during NREM and REM sleep were significantly different from that during alertness (P < 0.05, *), but not from each other.
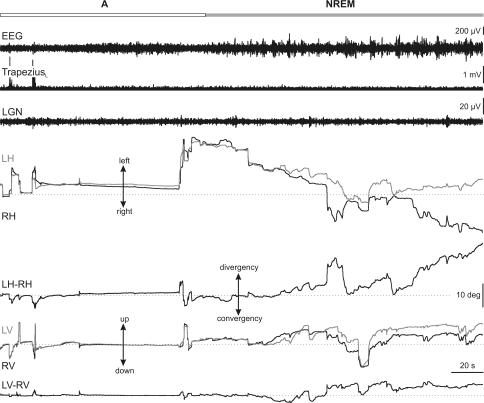
At the end of alertness, saccadic eye movements became smaller, and the eyes drifted slowly at random until the beginning of REM sleep (Fig. 3, right). During NREM sleep, the EEG was characterized by high-amplitude synchronized EEG, muscular tonus and absence of PGO waves. Eye movements during NREM sleep were characterized by low velocity and a varying degree of interocular disparity in the horizontal and vertical planes. In the horizontal plane, the eyes showed a divergent abducting movement with a mean interocular disparity of 4.8 ± 2.1 deg (n = 9) (Fig. 5). In the vertical plane, there was also an interocular disparity of −3.0 ± 3.3 deg (n = 9) (Fig. 5). When these values were compared with those obtained during alertness, significant differences were observed in the horizontal (P < 0.01; n = 9) and the vertical (P < 0.05; n = 9) planes (Fig. 5), showing that the interocular disparity was higher during NREM sleep than during alertness.
Figure 3. Eye movements and interocular disparities during NREM sleep.
Representative recording of the electroencephalographic (EEG) activity, rectified electromyographic activity of the left trapezius, and lateral geniculate nucleus (LGN) activity, together with left (grey traces) and right (black traces) horizontal (LH, RH) and vertical (LV, RV) eye position during non-rapid eye movement (NREM) sleep. Alertness (A) and NREM sleep (NREM) periods are demarcated by the horizontal bar at the top of the figure. During NREM sleep, eye position was characterized by low-velocity non-conjugated eye movements. Interocular disparity was displayed as a divergence in the horizontal (LH–RH) plane and a misalignment in the vertical (LV–RV) plane with respect to alertness. Dotted lines indicate zero value.
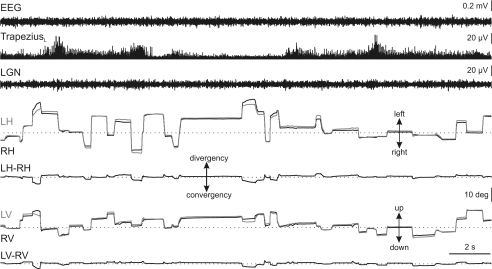
During transition periods, the visual axes of the two eyes began to converge slowly in the horizontal plane. This convergent adducting movement suddenly increased at the end of the transition period, and was present the whole time throughout REM sleep (Fig. 4). The mean interocular disparity in the horizontal plane during REM sleep was −12.0 ± 3.8 deg (n = 9). This value was significantly different (P < 0.01, n = 9) from that obtained during wakefulness and NREM sleep (Fig. 5). The converging position of the eyes was broken only by rapid divergent eye movements, although the eyes tended to drift quickly to the former convergent position after each rapid eye movement. In order to obtain a more reliable quantification of the interocular disparity, we also measured the maximum stabilized convergence. This was obtained as the value to which interocular disparity values tended or at which the convergence was maximum and remained stabilized. The maximum stabilized convergence value was 27.7 ± 6.8 deg (n = 9). In the vertical plane, both eyes tended to turn strongly downward and to maintain a downward position throughout REM sleep (Fig. 4). This downward eye position was broken only by some rapid upward eye movements, after each of which the eyes tended to return slowly to the previous downward position. The disparity between the two eyes in the vertical plane during REM sleep was 1.8 ± 1.9 deg (n = 9). This value was statistically different (P < 0.05) from that obtained during alertness, but not that during NREM sleep (Fig. 5). The mean of the maximum stabilized downward eye position to which the eyes tended during REM sleep was 25.5 ± 4.6 deg (n = 9).
Figure 4. Eye movements and interocular disparities during the transition period and REM sleep.
Representative recording of the electroencephalographic (EEG) activity, rectified electromyographic activity of the left trapezius, and lateral geniculate nucleus (LGN) activity, together with left (grey traces) and right (black traces) horizontal (LH, RH) and vertical (LV, RV) eye position during the transition period and REM sleep. The transition period (T) was identified by the occurrence of isolated ponto-geniculo-occipital waves in the presence of high-amplitude EEG activity and muscular tonus. During REM sleep, the right eye deviated to the left and the left eye to the right (curved arrows in horizontal eye-position traces), generating a net tonic convergence. Rapid eye movements in the horizontal plane were associated with PGO activities at the LGN, and induced a loss of the convergence, although almost never inducing a true divergence. In the vertical plane, the two eyes rotated downwardly (curved arrow in vertical eye-position traces) but with little modification of the interocular disparity in this plane. All rapid eye movements in the vertical plane were upwardly directed.
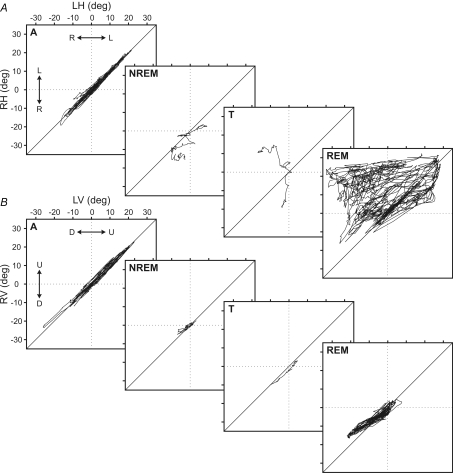
Figure 6 shows a graphical representation of the trajectories of the right eye versus the left in the horizontal (Fig. 6A) and the vertical (Fig. 6B) planes during a representative sleep–wake cycle in the cat. In these plots, the differences in eye position during alertness, NREM sleep, transition period and REM sleep phases can be seen clearly.
Figure 6. Interocular disparity plots for horizontal and vertical eye movements along the sleep–wake cycle.
Each square represents right versus left eye trajectories for horizontal (RH, LH) (A) and vertical (RV, LV) planes (B) along alertness (A), non-REM sleep (NREM), transition period (T), and REM sleep (REM) during one representative sleep–wake cycle in one cat. A, during alertness, eye movements were conjugated and their trajectory was distributed along the bisecting line. During REM and NREM sleep plots, eye positions were distributed over and under the bisecting line, showing a trend of tonic convergence and divergence, respectively. This reversion between divergence and convergence occurred during the transition period. B, in the vertical plane, trajectory points were similarly distributed along the bisecting line during the whole sleep–wake cycle, showing a lower interocular disparity in the vertical plane.
Phasic phenomena affecting the oculomotor system along the sleep–wake cycle
Phasic aspects in the oculomotor system during sleep occurred almost exclusively during the transition period and REM sleep; notwithstanding, a few low-velocity eye movements were present during NREM sleep (Fig. 3). Unfortunately, the scarcity of eye movements during NREM sleep makes their analysis impossible.
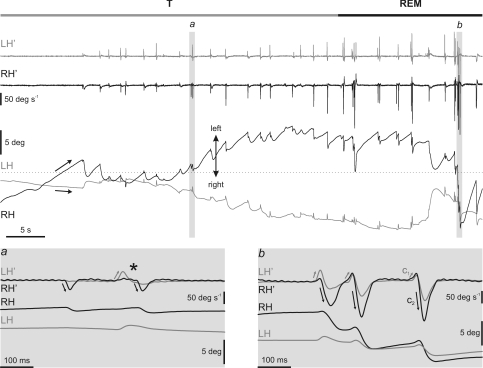
Phasic oculomotor activities developed slowly, starting with isolated eye movements of low amplitude and velocity during the transition period, which became grouped, bigger and faster as REM sleep progressed (Fig. 7). All rapid eye movements were associated with PGO waves, even during the transition period, and no PGO wave was recorded without its corresponding eye movement. Rapid eye movements during the transition period were simpler than those during REM sleep (insets in Fig. 7), where the most-complex and -intricate eye movements were recorded.
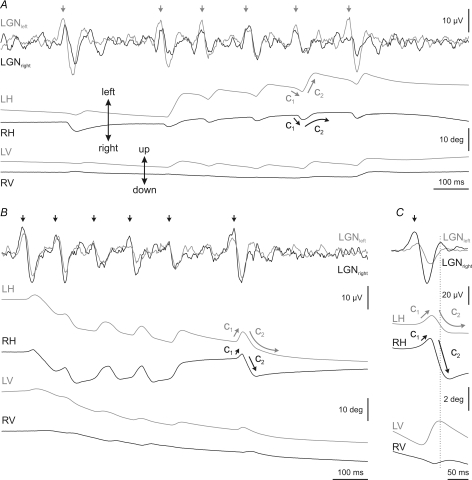
Figure 7. Qualitative differences between rapid eye movements in the transition period and REM sleep.
The figure shows a representative recording of the left (LH, grey) and right (RH, black) horizontal eye position and velocity (LH′ and RH′) during the transition period (T) and the beginning of REM sleep. Arrows on eye-position traces show the beginning of the strong tonic convergence during REM sleep. The left inset (a) at the bottom of the figure shows a detail of the characteristic monocular rapid eye movements during the transition period. Isolated rapid eye movements took place exclusively in the abducting directions. Black and grey arrows indicate rightward movement of the right eye and leftward movement of the left eye. Just before REM sleep, each abducting eye movement of one eye tended to pair at short latency with another abducting movement of the contralateral eye (asterisk). The right inset (b) shows three eye movements of a cluster in which each movement comprised two components (C1 and C2). The two components occurred in opposite directions, and C1 was of lower amplitude than C2. The maximum velocities of C1 and C2 were always in the eye that performed the abducting movement (grey and black arrows). Velocity traces in the insets are shown superimposed for easy comparison.
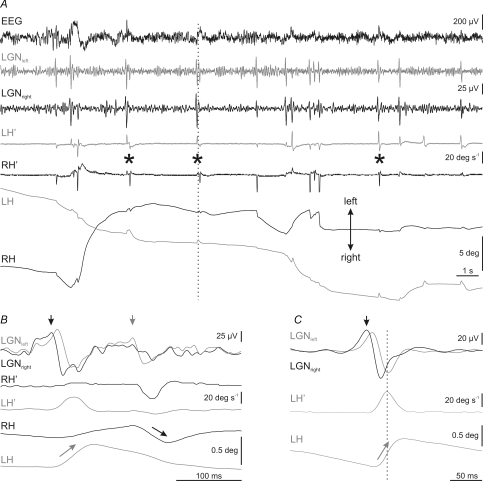
During the transition period, the majority of rapid eye movements occurred in the horizontal plane (Fig. 4). In this plane, rapid eye movements consisted of very small abducting movements, with amplitude ranging from 0.1 to 5 deg, and mainly affected the eye ipsilateral to the direction of movement (Fig. 7). Just before REM sleep, these monocular abducting eye movements tended to pair with another movement of the contralateral eye, also in the abducting direction (asterisks in inset a of Fig. 7 and in Fig. 8A). Thus, it was easy to see small monocular eye movements of the right eye to the right, followed at short latencies by movements of the left eye to the left, and vice versa. During each eye movement, a PGO wave was recorded at each LGN. The PGO wave contralateral to the side of rotation was always bigger than and occurred before (primary PGO) that at the ipsilateral LGN (secondary PGO) (Fig. 8B and C).
Figure 8. Temporal relationship between rapid eye movements and PGO waves during the transition period.
A, recording of electroencephalographic (EEG) activity, left (grey traces) and right (black traces) lateral geniculate nucleus (LGNleft, LGNright) activity, and horizontal eye velocity (LH′, RH′) and position (LH, RH) during a representative transition period. Rapid eye movements were associated to the occurrence of ponto-geniculo-occipital (PGO) waves at the LGN, and no PGO waves were recorded without the corresponding eye movement. Pairs of monocular abducting rapid eye movements of each eye are indicated by asterisks. B, expanded period at the time indicated by the vertical dotted line in A, showing a detail of LGN activities, and eye velocity and position during a pair of eye movements. Vertical arrows in LGN traces show the side (black, right; grey, left) on which primary PGO waves were recorded. Arrows on eye position indicate the eye that rotates in the abducting direction during primary PGO waves. C, average (n = 50) of left and right LGN activities during abducting movements of the left eye. The averaging was triggered by the peak velocity of the movement. Primary PGO wave, marked in this case by a black arrow, was always on the contralateral side with respect to the eye that performed the abducting movement.
During REM sleep, rapid eye movements occurred in clusters of up to nine consecutive high-velocity movements. The mean intracluster frequency, calculated as the inverse of the time interval between consecutive eye movements in the same cluster, was 7.4 ± 1.4 Hz (n = 200). Horizontal rapid eye movements during REM sleep were conjugated, although in most cases the movement of the eye ipsilateral to the direction of rotation was at higher velocity and amplitude than that of the contralateral one (Fig. 7). Thus, rapid eye movements tended to break the tonic convergent eye position, inducing a transient divergence of the visual axes (Fig. 4).
Each eye movement of the cluster in the horizontal plane comprised two components. The first component (C1 in inset b of Fig. 7 and Fig. 9) was a very brief eye movement that was immediately followed by a bigger second component (C2 in inset b of Fig. 7 and Fig. 9) directed to the contralateral side. The latency between C1 and C2 was 22.7 ± 5.6 ms (n = 150). As a rule, the velocity of each component was higher in the eye ipsilateral to the direction of the movement. Thus, if the first component was directed rightward, it was faster in the right eye, and the second component was directed leftward and was faster in the left eye (Fig. 9). In addition to the difference in velocity between the two components, the second component tended to maintain the position reached by the ipsilateral eye (straight grey arrow in Fig. 9A and black one in Fig. 9B and C at C2 component), whereas the ipsilateral eye came back slowly to its position before the first component (curved black arrow in Fig. 9A and grey one in Fig. 9B and C at C2 component).
Figure 9. Temporal relationship between rapid eye movements and PGO waves during REM sleep.
Recording of left (grey) and right (black) lateral geniculate nucleus (LGNleft, LGNright) activities and horizontal (LH, RH) and vertical (LV, RV) eye position during representative clusters of eye movements in REM sleep. LGN activities are shown superimposed for easy comparison. A, during a cluster of rapid eye movements in which the first component (C1) was directed rightward and the second (C2) leftward, primary (vertical grey arrows) and secondary PGO waves were recorded at the left and right LGN, respectively. B, during clusters in which the first component (C1) was directed leftward, primary PGO waves (vertical black arrows) were recorded at the left LGN. C, averaging (n = 50) of LGN activities and eye position in horizontal and vertical planes triggered by the peak velocity of the right eye during the second component. The primary PGO wave took place at the right LGN. Note that during the C2 component, only the eye that performed the abducting movement tended to maintain the position reached, whereas the other eye drifted back to the position that it had before. In the vertical plane, eye movements during PGO waves displayed only one component, which was always upwardly directed.
During the occurrence of each rapid eye movement, a PGO wave was recorded at each LGN (Fig. 9). Primary PGO waves (vertical grey arrows in Fig. 9A and black arrows in Fig. 9B on the LGN traces) occurred at the LGN contralateral to the one the first component of eye movements was directed at (C1 in Fig. 9A–C). Thus, the biggest eye movements that occurred during the second component were directed to the side on which the secondary PGO wave was recorded at the LGN. During a cluster, the direction of rotation of the two components for each eye movement was identical, and primary PGO waves were always recorded at the same LGN. Figure 9C shows an average (n = 50) of PGO waves and the eye position in the horizontal and vertical planes triggered by the peak velocity of the rightward-directed C2 component of the movement.
In the vertical plane, rapid upward movements coincided with the occurrence of horizontal rapid eye movements. Vertical eye movements were always upwardly directed, independently of the direction of rapid eye movements in the horizontal plane. These upward movements were scarcer and slower than the divergent ones in the horizontal plane, and the eyes very rarely passed the centre of the orbit (Fig. 4).
Peak eye velocity–amplitude relationship during alertness, transition period and REM sleep
With the aim of testing possible differences in the codification of saccadic eye movements during alertness and the different rapid eye movements observed during the transition and the REM sleep phases, the relationship between peak eye velocity and amplitude was analysed.
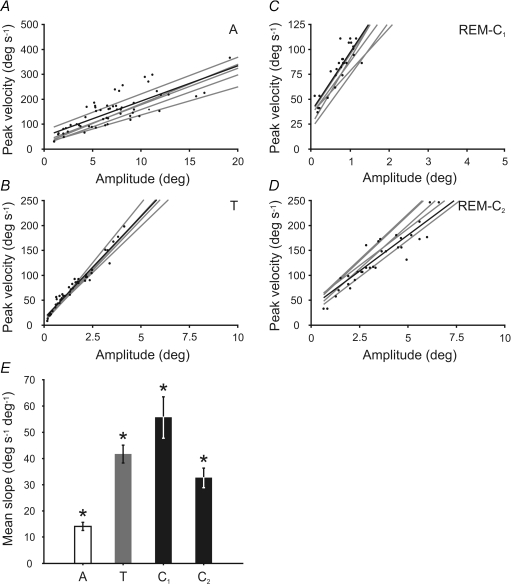
In the horizontal plane, the mean slope of the relationship between eye velocity and amplitude during alertness was 14.1 ± 1.6 deg s−1 deg−1 (n = 6) (Fig. 10A). During the NREM–REM sleep transition period, the mean slope for the relationship between eye velocity and amplitude was 41.7 ± 3.4 deg s−1 deg−1 (n = 6) (Fig. 10B), while during REM sleep, the mean slope was 55.7 ± 8.0 deg s−1 deg−1 (n = 6) and 32.7 ± 3.7 deg s−1 deg−1 (n = 6) for the first (Fig. 10C) and second component (Fig. 10D), respectively. Thus, the mean slope of the velocity–amplitude relationship was lesser during alertness than during sleep, and that during the NREM–REM sleep transition was intermediate. During REM sleep, the mean slope of the first component was greater than that of the second. All comparisons were statistically significant (P < 0.01) (asterisks in Fig. 10E).
Figure 10. Comparison of rapid eye movement during alertness and sleep.
Relationship between peak eye velocity and amplitude in the horizontal plane during saccadic eye movements in alertness (A), rapid eye movement in the transition period (B), and during the first (C) and second (D) components of rapid eye movements during REM sleep. Data for a representative period and their corresponding regression line are plotted in black. Grey lines represent regression lines for other analysed episodes in each period. The scales of the plots have been proportionally modified to facilitate slope comparison. E, mean slope comparison for each type of rapid eye movement during 6 sleep–wake cycles. Asterisk on a bar indicates the existence of statistical differences with the rest of the bars (P < 0.01).
In the vertical plane, the mean slope of the velocity–amplitude relationship was 14.5 ± 2.1 deg s−1 deg−1 (n = 6) during alertness, and 18.9 ± 3.7 deg s−1 deg−1 (n = 6) for rapid upward movement during REM sleep. Despite the apparent similarity of these mean values, they were significantly different when compared (P < 0.05; n = 6).
Discussion
Since the first report of the existence of rapid eye movements during sleep (Aserinsky & Kleitman, 1953), many authors have tried to characterize the origin of this motor activity and its relationship with other REM sleep phenomena (Bizzi et al. 1964; Jeannerod et al. 1965; Sakai & Cespuglio, 1976; Hoshino et al. 1976; Sakai et al. 1976; Pivik et al. 1977; Nelson et al. 1983; Datta & Hobson, 1994; Vanni-Mercier et al. 1996; Vanni-Mercier & Debilly, 1998), its dynamic properties (Fuchs & Ron, 1968; Bon et al. 1980; Herman et al. 1983; Aserinsky et al. 1985; Vanni-Mercier et al. 1994; Escudero & Vidal, 1996; Zhou & King, 1997), and its possible linkage with oneiric imagery (Aserinsky & Kleitman, 1955; Dement & Kleitman, 1957; Herman et al. 1983; Aserinsky et al. 1985; Zhou & King, 1997). Nevertheless, present knowledge on eye movements during sleep is incomplete, and the literature contains some contradictory conclusions about the relationship between rapid eye movements and PGO waves during REM sleep.
In this paper, the analysis of eye movement during sleep has revealed that the oculomotor system is driven by tonic and phasic activities. The quantitative analysis of tonic signals shows that the direction of eye movements during NREM and REM sleep are opposed. With regard to phasic signals, we show the existence of single rapid eye movements during the transition period and complex two-component rapid eye movements during REM sleep, and their relationship to PGO waves at the LGN.
Tonic activities during NREM sleep
During NREM sleep, the eyes moved erratically and slowly, as previously described in humans (Aserinsky & Kleitman, 1953, 1955), monkeys (Fuchs & Ron, 1968; Henn et al. 1984), cats (Fukushima & Fukushima, 1990; Vanni-Mercier et al. 1994) and guinea pigs (Escudero & Vidal, 1996). Moreover, we consistently observed a tonic divergence of the two eyes in the horizontal plane during NREM sleep. A tonic divergence of the eyes in the horizontal plane requires the activation of the lateral recti and the deactivation of the medial recti muscles. However, an activation mechanism seems improbable, because a previous work in which ABD motoneurons were recorded during NREM sleep has shown a firing rate decrease (Henn et al. 1984). Another extraocular muscle that could produce a divergence is the inferior oblique. Although the primary action of the inferior oblique muscle is extorsion, it also produces abduction and elevation as secondary actions, and elevation was observed occasionally during NREM sleep (Fuchs & Ron, 1968; Bon et al. 1980). However, it is more probable that the mechanism for divergence and elevation of the eyes during NREM sleep is a deactivation of the oculomotor system at both motor and premotor levels. Although the cat is considered a frontal-eyed species, its eyes are slightly lateral, and some co-contraction of the medial recti is necessary to maintain good binocular vision during alertness, as reflected by the pupillary divergence during the first weeks in kittens that becomes convergent visual axes in adults (Olson & Freeman, 1978), or its maintained divergence when the animals are reared in the dark (Cynader, 1979). Interestingly, Cynader (1979) reported a mean divergence of 4.6 deg in dark-reared cats with respect to control animals, a value very similar to that obtained in the present results during NREM sleep (4.8 deg). A similar reasoning could be applied for the vertical plane, in which some active downward movement is necessary for near perception during alertness. Thus, a decrease in the activity of extraocular muscles during NREM sleep could induce a divergence and elevation of the eyes with respect to alertness. In this sense, a decrease of the firing rate has previously been reported in the ABD (Henn et al. 1984) and medial rectus motoneurons (De la Cruz et al. 1989), and in neurons of the premotor extraocular system (Henn et al. 1984).
Tonic activities during the transition period and REM sleep
A previous work (Zhou & King, 1997) in monkeys reported the existence of misalignment between the eyes during REM sleep. However, those authors did not report how the misalignment varied along sleep. We have found a downward movement and a convergence of the eyes in the vertical and horizontal planes, respectively, that developed during the transition period and consolidated during REM sleep. These eye movements were very strong in comparison with the vergence and elevation of the eyes during NREM sleep, and probably require active mechanisms to generate them.
Vertical downward movement requires the activation of the inferior rectus and superior oblique muscles, and deactivation of the superior rectus and inferior oblique. The superior rectus is closely associated embryologically and developmentally with the levator palpebrae muscle, and the two muscles share a common epimysium (Sevel, 1986). Furthermore, the levator palpebrae motoneurons lie adjacent to those of the superior rectus, with which they seem to share common premotor inputs (Horn et al. 2000; Chen & May, 2002; Morcuende et al. 2002). Therefore, a deactivation of the superior rectus could be part of the same mechanism that inhibits the levator palpebrae motoneurons, provoking the closing of the eyelid during sleep. However, as this deactivation is probably insufficient to explain the strong downward movement, a tonic activation of the inferior rectus muscle must also be considered. In coherence with this, Orem & Dement (1974) reported that the lower lid underwent a tonic descent during REM sleep. Although the lower lid is less associated to the inferior rectus, the strong downward movement of the eye could be responsible for this depression of the lower lid.
Tonic convergence in the horizontal plane requires the deactivation of the lateral rectus and/or the activation of the medial rectus muscle of each eye. The lateral rectus seems to be deactivated because ABD motoneurons are tonically inhibited during REM sleep (Escudero & Márquez-Ruiz, 2008). Medial rectus motoneurons are mainly controlled by internuclear interneurons in the contralateral ABD nucleus (Highstein et al. 1982) and by second-order vestibular neurons through the tract of Deiters (Reisine et al. 1981; McCrea et al. 1987). As internuclear interneurons in the ABD nucleus seem to receive similar inputs to those of ABD motoneurons, it seems improbable that they could be responsible for convergence during REM sleep. By contrast, second-order vestibular neurons seem to maintain their tonic firing discharge during REM sleep (Bizzi et al. 1964), and could be responsible for tonic signals in medial rectus motoneurons.
Phasic activities during transition from NREM to REM sleep
Some authors coincide in defining a brief phase that precedes REM sleep, named ‘slow wave associated to PGO waves’ or ‘transition period’. Its segregation from REM sleep is supported by changes produced in EEG activity (McCarley & Hobson, 1970; Trachsel et al. 1988; Glin et al. 1991; Gottesmann, 1992) and the occurrence of isolated PGO waves (Steriade et al. 1989; Datta & Hobson, 1994; Datta, 1997). The results shown in the present work demonstrate the existence of qualitative differences between rapid eye movements produced during the transition period and REM sleep. These differences allow the effective separation of the two states.
During the transition period, rapid eye movements were restricted to the horizontal plane, and their velocity and amplitude increased gradually. They were isolated, mainly monocular and in the abducting direction. Each eye movement was associated with a PGO wave, and no isolated PGO wave was observed without its corresponding eye movement. These results contradict those of previous studies (Thomas & Benoit, 1967; Gottesmann, 1996) reporting the existence of PGO waves without eye movements. It is probable that the low amplitude of such eye movements during the transition period is missed by the EOG technique used in those studies. Isolated eye movements during the transition period contrasted with the more-complex two-component eye movements associated with grouped PGO waves during REM sleep. At the end of the transition period, monocular eye movements began to become organized. Each monocular movement was followed by another movement of the contralateral eye at a latency that decreased progressively until producing opposed-direction coupled eye movements just before REM sleep. This temporal sequence allows postulation of the existence of two independent phasic signal generators that work independently at the beginning of the transition period but tend to organize as the transition period progresses, finalizing in a tight coupling during REM sleep. Such a hypothesis is supported by the consistent occurrence of primary PGO waves at the contralateral LGN with respect to the direction of eye movements during the transition period and the first component of the eye movement during REM sleep.
Phasic activities during REM sleep
During REM sleep, eye movements were mainly horizontal and grouped in high-frequency clusters. In the horizontal plane, each movement in the cluster comprised two components and was associated with a PGO wave. The two components moved the eyes in opposite directions without any latency in between. These rapid back-and-forth eye movements grouped in clusters recall the eye movements observed in guinea pigs during REM sleep (Escudero & Vidal, 1996). As these eye movements are exclusive to REM sleep, motor commands underlying rapid eye movements during REM sleep seem not to be the same that generate saccades during alertness. In fact, latency between the two components was shorter than that reported for the organization of saccadic and even saccadic express eye movements (Fischer & Ramsperger, 1984; Becker, 1989). These data do not support, at least for rapid eye movements, the proposed scanning hypothesis (Aserinsky & Kleitman, 1955; Herman et al. 1983; Aserinsky et al. 1985) that associates rapid eye movements with visual dream scanning.
Most authors agree that rapid eye movements during REM sleep are directed to the side where the primary PGO wave is recorded (Cespuglio et al. 1975; Sakai & Cespuglio, 1976; Sakai et al. 1976; Nelson et al. 1983). We found that primary PGO waves occurred at the LGN contralateral to the one at which the first component was directed and ipsilateral to the second component. Thus, it is likely that previous works refer to the second component of eye movement, which is higher in amplitude than the first component. As mentioned previously, it is probable that the low spatial resolution techniques used in most of the papers has meant that the first component has hitherto passed unnoticed.
With regard to muscular activity, vertical upward movements require a phasic activation of the superior rectus and/or inferior oblique muscle. The fact that upward movements usually did not pass the centre of the orbit probably rules out eyelid raising as the origin of these movements, because rapid twitches of the upper lid during REM sleep are of low amplitude (Orem & Dement, 1974). Another possible source of rapid vertical eye movements is the activation of the accessory ABD motoneurons. These motoneurons innervate the retractor bulbi, a muscle constituted of four strips originating from a common tendon, inserted behind the four recti muscles, and whose function is to retract the eye, allowing the closing of the nictitating membrane and of the eyelid. Thus, if the eye is depressed, a phasic activation of the retractor bulbi muscle could induce a vertical upward movement that does not pass the centre of the orbit (Delgado-García et al. 1990). In the horizontal plane, abducting movements were always greater than adducting ones. This result perhaps indicates that phasic activation is acting on the ABD but not on the oculomotor nucleus, which would not be surprising, because each ABD nucleus constitutes the final output for ipsilateral conjugated horizontal eye movements (Escudero & Delgado-García, 1988; Escudero et al. 1992).
Peak eye velocity–amplitude relationship during alertness, the transition period and REM sleep
The velocity–amplitude relationship of rapid eye movements during REM sleep is one of the most controversial areas in the literature. Previous works have reported that compared with saccades during alertness, rapid eye movements during REM sleep are slower (Fuchs & Ron, 1968; Aserinsky et al. 1985), of equal velocity (Herman et al. 1983; Zhou & King, 1997), or faster (Vanni-Mercier et al. 1994; Escudero & Vidal, 1996). Present results demonstrate the existence of three different types of rapid eye movement in the horizontal plane during sleep: isolated monocular eye movements during the transition period, and first and second components of conjugated rapid eye movement during REM sleep. The analysis of the relationship between peak velocity and amplitude for these eye movements yielded a higher slope in all three types of movement during sleep with respect to alertness. The slope for the secondary component of eye movements during REM sleep agrees with the value obtained by Vanni-Mercier et al. (1994) using the same eye-movement recording technique in monocularly implanted cats. The other two types of rapid eye movement have not been described until now.
During REM sleep, the highest slope was obtained for the first component, followed by isolated eye movements during the transition period and then by the second component during REM sleep. Without recording the activity of motor and premotor extraocular neurons, it is not easy to explain why slopes are higher during sleep than during alertness, but it is probable that the synchronic discharge of almost all motoneurons is the basis of higher slopes. Thus, as the first component during REM sleep and monocular eye movements during the transition period were the shortest, probably the synchronicity of motoneurons was highest, explaining the higher slopes for these eye movements.
The tonic and phasic characteristics in eye movements described in this paper recall some aspects of the tonic inhibition and phasic activations and inhibitions described in other motor system during REM sleep (Nakamura et al. 1978; Glenn et al. 1978). Notwithstanding, the full establishment of this parallelism requires knowing the activity of extraocular motoneurons during REM sleep. In a companion paper (Escudero & Márquez-Ruiz, 2008) we describe the activity of ABD motoneurons during sleep and we demonstrate the similarity between the mechanisms operating on extraocular motoneurons and other motor neurons during REM sleep.
Acknowledgments
This research was supported by grant BFU2005-01579 from the Ministerio de Educación y Ciencia and by the Consejería de Innovación, Ciencia y Empresa of the Junta de Andalucía, Spain. We would like to thank Mr Roger Churchill for editorial help.
References
- Aserinsky A, Kleitman N. Regularly occurring periods of eye motility, and concomitant phenomena, during sleep. Science. 1953;118:273–274. doi: 10.1126/science.118.3062.273. [DOI] [PubMed] [Google Scholar]
- Aserinsky A, Kleitman N. Two types of ocular motility occurring in sleep. J Appl Physiol. 1955;8:1–10. doi: 10.1152/jappl.1955.8.1.1. [DOI] [PubMed] [Google Scholar]
- Aserinsky E, Lynch JA, Mack ME, Tzankoff SP, Hurn E. Comparison of eye motion in wakefulness and REM sleep. Psychophysiology. 1985;22:1–10. doi: 10.1111/j.1469-8986.1985.tb01551.x. [DOI] [PubMed] [Google Scholar]
- Becker W. Metrics. In: Wurtz RH, Goldberg ME, editors. The Neurobiology of Saccadic Eye Movements. Amsterdam: Elsevier; 1989. pp. 13–67. [PubMed] [Google Scholar]
- Bizzi E, Pompeiano O, Somogyi I. Vestibular nuclei: activity of single neurons during natural sleep and wakefulness. Science. 1964;145:414–415. doi: 10.1126/science.145.3630.414. [DOI] [PubMed] [Google Scholar]
- Bon L, Corazza R, Inchingolo P. Eye movements during the waking-sleep cycle of the encephale isole semichronic cat preparation. Electroencephalogr Clin Neurophysiol. 1980;48:327–340. doi: 10.1016/0013-4694(80)90269-2. [DOI] [PubMed] [Google Scholar]
- Cespuglio R, Laurent JP, Jouvet M. Etude des relations entre l'activité ponto-geniculo-occipitale (PGO) et la motricité oculaire chez le chat sous réserpine. Brain Res. 1975;83:319–335. doi: 10.1016/0006-8993(75)90939-7. [DOI] [PubMed] [Google Scholar]
- Chase MH, Morales FR. Subthreshold excitatory activity and motoneuron discharge during REM periods of active sleep. Science. 1983;221:1195–1198. doi: 10.1126/science.6310749. [DOI] [PubMed] [Google Scholar]
- Chen B, May PJ. Premotor circuits controlling eyelid movements in conjunction with vertical saccades in the cat. II. Interstitial nucleus of Cajal. J Comp Neurol. 2002;500:676–692. doi: 10.1002/cne.21203. [DOI] [PubMed] [Google Scholar]
- Cynader M. Interocular alignment following visual deprivation in the cat. Invest Ophthalmol Vis Sci. 1979;18:726–741. [PubMed] [Google Scholar]
- Datta S. Cellular basis of pontine ponto-geniculo-occipital wave generation and modulation. Cell Mol Neurobiol. 1997;17:341–365. doi: 10.1023/a:1026398402985. [DOI] [PubMed] [Google Scholar]
- Datta S, Hobson JA. Neuronal activity in the caudolateral peribrachial pons: relationship to PGO waves and rapid eye movements. J Neurophysiol. 1994;71:95–109. doi: 10.1152/jn.1994.71.1.95. [DOI] [PubMed] [Google Scholar]
- De la Cruz RR, Escudero M, Delgado-García JM. Behaviour of medial rectus motoneurons in the alert cat. Eur J Neurosci. 1989;1:288–295. doi: 10.1111/j.1460-9568.1989.tb00796.x. [DOI] [PubMed] [Google Scholar]
- Delgado-García JM, Evinger C, Escudero M, Baker R. Behavior of accessory abducens and abducens motoneurons during eye retraction and rotation in the alert cat. J Neurophysiol. 1990;64:413–422. doi: 10.1152/jn.1990.64.2.413. [DOI] [PubMed] [Google Scholar]
- Dement W. The occurrence of low voltage, fast electroencephalogram patterns during behavioral sleep in the cat. Electroencephalogr Clin Neurophysiol. 1958;10:291–296. doi: 10.1016/0013-4694(58)90037-3. [DOI] [PubMed] [Google Scholar]
- Dement W, Kleitman N. The relation of eye movements during sleep to dream activity: an objective method for the study of dreaming. J Exp Psychol. 1957;53:339–346. doi: 10.1037/h0048189. [DOI] [PubMed] [Google Scholar]
- Escudero M, de la Cruz RR, Delgado-García JM. A physiological study of vestibular and prepositus hypoglossi neurones projecting to the abducens nucleus in the alert cat. J Physiol. 1992;458:539–560. doi: 10.1113/jphysiol.1992.sp019433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudero M, Delgado-García JM. Behavior of reticular, vestibular and prepositus neurons terminating in the abducens nucleus of the alert cat. Exp Brain Res. 1988;71:218–222. doi: 10.1007/BF00247538. [DOI] [PubMed] [Google Scholar]
- Escudero M, Márquez-Ruiz J. Tonic inhibition and ponto-geniculo-occipital-related activities shape abducens motoneuron discharge during REM sleep. J Physiol. 2008;586:3479–3491. doi: 10.1113/jphysiol.2008.153254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escudero M, Vidal PP. A quantitative study of electroencephalography, eye movements and neck electromyography characterizing the sleep-wake cycle of the guinea-pig. Eur J Neurosci. 1996;8:572–580. doi: 10.1111/j.1460-9568.1996.tb01242.x. [DOI] [PubMed] [Google Scholar]
- Fischer B, Ramsperger E. Human express saccades: extremely short reaction times of goal directed eye movements. Exp Brain Res. 1984;57:191–195. doi: 10.1007/BF00231145. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Robinson DA. A method for measuring horizontal and vertical eye movement chronically in the monkey. J Appl Physiol. 1966;21:1068–1070. doi: 10.1152/jappl.1966.21.3.1068. [DOI] [PubMed] [Google Scholar]
- Fuchs AF, Ron S. An analysis of rapid eye movements of sleep in the monkey. Electroencephalogr Clin Neurophysiol. 1968;25:244–251. doi: 10.1016/0013-4694(68)90022-9. [DOI] [PubMed] [Google Scholar]
- Fukushima K, Fukushima J. Activity of eye-movement-related neurons in the region of the interstitial nucleus of Cajal during sleep. Neurosci Res. 1990;9:126–139. doi: 10.1016/0168-0102(90)90028-d. [DOI] [PubMed] [Google Scholar]
- Glenn LL, Foutz AS, Dement WC. Membrane potential of spinal motoneurons during natural sleep in cats. Sleep. 1978;1:199–204. [PubMed] [Google Scholar]
- Glin L, Arnaud C, Berracochea D, Galey D, Jaffard R, Gottesmann C. The intermediate stage of sleep in mice. Physiol Behav. 1991;50:951–953. doi: 10.1016/0031-9384(91)90420-s. [DOI] [PubMed] [Google Scholar]
- Gottesmann C. Detection of seven sleep-waking stages in the rat. Neurosci Biobehav Rev. 1992;16:31–38. doi: 10.1016/s0149-7634(05)80048-x. [DOI] [PubMed] [Google Scholar]
- Gottesmann C. The transition from slow-wave sleep to paradoxical sleep: evolving facts and concepts of the neurophysiological processes underlying the intermediate stage of sleep. Neurosci Biobehav Rev. 1996;20:367–387. doi: 10.1016/0149-7634(95)00055-0. [DOI] [PubMed] [Google Scholar]
- Henn V, Baloh RW, Hepp K. The sleep-wake transition in the oculomotor system. Exp Brain Res. 1984;54:166–176. doi: 10.1007/BF00235828. [DOI] [PubMed] [Google Scholar]
- Herman JH, Barker DR, Roffwarg HP. Similarity of eye movement characteristics in REM sleep and the awake state. Psychophysiology. 1983;20:537–543. doi: 10.1111/j.1469-8986.1983.tb03008.x. [DOI] [PubMed] [Google Scholar]
- Herman JH, Erman M, Boys R, Peiser L, Taylor ME, Roffwarg HP. Evidence for a directional correspondence between eye movements and dream imagery in REM sleep. Sleep. 1984;7:52–63. doi: 10.1093/sleep/7.1.52. [DOI] [PubMed] [Google Scholar]
- Highstein SM, Karabelas A, Baker R, McCrea RA. Comparison of the morphology of physiologically identified abducens motor and internuclear neurons in the cat: a light microscopic study employing the intracellular injection of horseradish peroxidase. J Comp Neurol. 1982;208:369–381. doi: 10.1002/cne.902080407. [DOI] [PubMed] [Google Scholar]
- Horn AK, Buttner-Ennever JA, Gayde M, Messoudi A. Neuroanatomical identification of mesencephalic premotor neurons coordinating eyelid with upgaze in the monkey and man. J Comp Neurol. 2000;420:19–34. doi: 10.1002/(sici)1096-9861(20000424)420:1<19::aid-cne2>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Hoshino K, Pompeiano O, Magherini PC, Mergner T. The oscillatory system responsible for the oculomotor activity during the bursts of REM. Arch Ital Biol. 1976;114:278–309. [PubMed] [Google Scholar]
- Jeannerod M, Mouret J, Jouvet M. Étude de la motricité oculaire au cours de la phase paradoxale du sommeil chez le chat. Electroencephalogr Clin Neurophysiol. 1965;18:554–566. doi: 10.1016/0013-4694(65)90073-8. [DOI] [PubMed] [Google Scholar]
- Jouvet M, Michel F. Corrélations électromyographique du sommeil chez le chat décortiqué et mesencéphalique chronique. C R Seances Soc Biol Fil. 1959;153:422–425. [PubMed] [Google Scholar]
- McCarley RW, Hobson JA. Cortical unit activity in desynchronized sleep. Science. 1970;167:901–903. doi: 10.1126/science.167.3919.901. [DOI] [PubMed] [Google Scholar]
- McCrea RA, Strassman A, May E, Highstein SM. Anatomical and physiological characteristics of vestibular neurons mediating the horizontal vestibulo-ocular reflex of the squirrel monkey. J Comp Neurol. 1987;264:547–570. doi: 10.1002/cne.902640408. [DOI] [PubMed] [Google Scholar]
- Morcuende S, Delgado-García JM, Ugolini G. Neuronal premotor networks involved in eyelid responses: retrograde transneuronal tracing with rabies virus from the orbicularis oculi muscle in the rat. J Neurosci. 2002;22:8808–8818. doi: 10.1523/JNEUROSCI.22-20-08808.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Goldberg LJ, Chandler SH, Chase MH. Intracellular analysis of trigeminal motoneuron activity during sleep in the cat. Science. 1978;199:204–207. doi: 10.1126/science.202025. [DOI] [PubMed] [Google Scholar]
- Nelson JP, McCarley RW, Hobson JA. REM sleep burst neurons, PGO waves, and eye movement information. J Neurophysiol. 1983;50:784–797. doi: 10.1152/jn.1983.50.4.784. [DOI] [PubMed] [Google Scholar]
- Olson CR, Freeman RD. Eye alignment in kittens. J Neurophysiol. 1978;41:848–859. doi: 10.1152/jn.1978.41.4.848. [DOI] [PubMed] [Google Scholar]
- Orem J, Dement WC. Spontaneous eyelid behavior in the sleeping cat. Exp Neurol. 1974;44:145–159. doi: 10.1016/0014-4886(74)90055-7. [DOI] [PubMed] [Google Scholar]
- Pivik RT, McCarley RW, Hobson JA. Eye movement-associated discharge in brain stem neurons during desynchronized sleep. Brain Res. 1977;121:59–76. doi: 10.1016/0006-8993(77)90438-3. [DOI] [PubMed] [Google Scholar]
- Reisine H, Strassman A, Highstein SM. Eye position and head velocity signals are conveyed to medial rectus motoneurons in the alert cat by the ascending tract of Deiters'. Brain Res. 1981;211:153–157. doi: 10.1016/0006-8993(81)90075-5. [DOI] [PubMed] [Google Scholar]
- Roffwarg HP, Dement WC, Muzio JN, Fisher C. Dream imagery: relationship to rapid eye movements of sleep. Arch Gen Psychiatry. 1962;7:235–258. doi: 10.1001/archpsyc.1962.01720040001001. [DOI] [PubMed] [Google Scholar]
- Sakai K, Cespuglio R. Evidence for the presence of eye movement potentials during paradoxical sleep in cats. Electroencephalogr Clin Neurophysiol. 1976;41:37–48. doi: 10.1016/0013-4694(76)90213-3. [DOI] [PubMed] [Google Scholar]
- Sakai K, Petitjean F, Jouvet M. Effects of ponto-mesencephalic lesions and electrical stimulation upon PGO waves and EMPs in unanesthetized cats. Electroencephalogr Clin Neurophysiol. 1976;41:49–63. doi: 10.1016/0013-4694(76)90214-5. [DOI] [PubMed] [Google Scholar]
- Sevel D. The origins and insertions of the extraocular muscles: development, histologic features, and clinical significance. Trans Am Ophthalmol Soc. 1986;84:488–526. [PMC free article] [PubMed] [Google Scholar]
- Steriade M. Grouping of brain rhythms in corticothalamic systems. Neuroscience. 2006;137:1087–1106. doi: 10.1016/j.neuroscience.2005.10.029. [DOI] [PubMed] [Google Scholar]
- Steriade M, McCarley RW. Brain Control of Wakefulness and Sleep. New York: Kluwer Academic-Plenum Publishers; 2005. [Google Scholar]
- Steriade M, Paré D, Bouhassira D, Deschènes M, Oakson G. Phasic activation of lateral geniculate and perigeniculate thalamic neurons during sleep with ponto-geniculo-occipital waves. J Neurosci. 1989;9:2215–2229. doi: 10.1523/JNEUROSCI.09-07-02215.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J, Benoit O. Individualisation d'un sommeil à ondes lentes et activité phasique. Brain Res. 1967;5:221–235. doi: 10.1016/0006-8993(67)90088-1. [DOI] [PubMed] [Google Scholar]
- Trachsel L, Tobler I, Borbely AA. Electroencephalogram analysis of non-rapid eye movement sleep in rats. Am J Physiol. 1988;255:27–37. doi: 10.1152/ajpregu.1988.255.1.R27. [DOI] [PubMed] [Google Scholar]
- Vanni-Mercier G, Debilly G. A key role for the caudoventral pontine tegmentum in the simultaneous generation of eye saccades in bursts and associated ponto-geniculo-occipital waves during paradoxical sleep in the cat. Neuroscience. 1998;86:571–585. doi: 10.1016/s0306-4522(98)00045-1. [DOI] [PubMed] [Google Scholar]
- Vanni-Mercier G, Debilly G, Lin JS, Pelisson D. The caudo ventral pontine tegmentum is involved in the generation of high velocity eye saccades in bursts during paradoxical sleep in the cat. Neurosci Lett. 1996;213:127–131. doi: 10.1016/0304-3940(96)12832-9. [DOI] [PubMed] [Google Scholar]
- Vanni-Mercier G, Pelisson D, Goffart L, Sakai K, Jouvet M. Eye saccade dynamics during paradoxical sleep in the cat. Eur J Neurosci. 1994;6:1298–1306. doi: 10.1111/j.1460-9568.1994.tb00320.x. [DOI] [PubMed] [Google Scholar]
- Zhou W, King WM. Binocular eye movements not coordinated during REM sleep. Exp Brain Res. 1997;117:153–160. doi: 10.1007/s002210050209. [DOI] [PubMed] [Google Scholar]