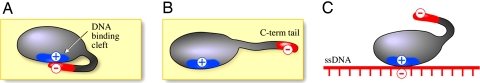
Fig. 1.
Model for the binding of ssDNA-binding protein to polynucleotides. Based on the studies of the binding of gp32 to various oligo- and polynucleotide substrates, Kowalczykowski et al. (17) proposed that the binding to polynucleotides is accompanied by a conformational change that results in “the pulling apart of a cluster of positively charged residues on the protein that have previously comprised tight anion binding site.” Thus, basic amino acid residues that have been shielded become available for nucleic acid binding. (A) Free protein. Negatively charged residues from C-terminal tail (in red) are contacting positively charged residues (in blue) via electrostatic interactions. (B) Displacement of the C-terminal tail results in the highly accessible DNA-binding cleft. (C) Bound state. The C-terminal tail is displaced, and previously shielded basic residues contact electrostatically the phosphate backbone (in red).