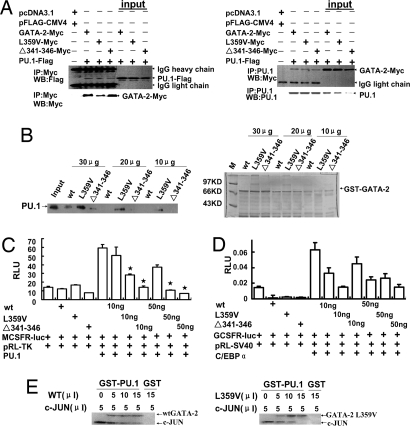
Fig. 3.
GATA-2 mutants exhibit enhanced inhibition on the transcriptional activity of PU.1. (A) GATA-2 mutants physically associate with PU.1 in vivo. Flag-tagged PU.1 cDNA plus one of three c-Myc-tagged GATA-2 cDNAs were cotransfected into Cos-7 cells. Left shows that the immunoprecipitation of whole-cell lysates by anti-Myc antibodies coprecipitates the PU.1 protein. Right shows that, in a reverse way, PU.1 antibody-mediated precipitates contain three GATA-2 proteins, as probed by an anti-c-Myc antibody. (B Left) Different doses of GST-wtGATA-2, GST-GATA-2 L359V, or GST-Δ341–346 mutant and GST were used to pull down the lysates of the PU.1-transfected Cos-7 cells. (B Right) Coomassie blue staining confirms the quantification of GST fusion proteins by Bradford assay. (C) The M-CSFR promoter-coupled luciferase reporter plasmid was cotransfected into Cos-7 cells, with different doses of wtGATA-2 or GATA-2 mutant expression plasmids as indicated. The relative luciferase units are expressed as mean ± SD. *, P < 0.05. (D) G-CSFR promoter-coupled luciferase reporter was cotransfected with different doses of plasmids as shown in C. (E) GATA-2 L359V competes with c-JUN on binding to PU.1. Combinations of different amounts of 35S-labeled GATA-2 and c-JUN as indicated were incubated with the GST-PU.1 protein. Pull-down efficiencies of GATA-2 and c-JUN by PU.1 were examined by autoradiography.