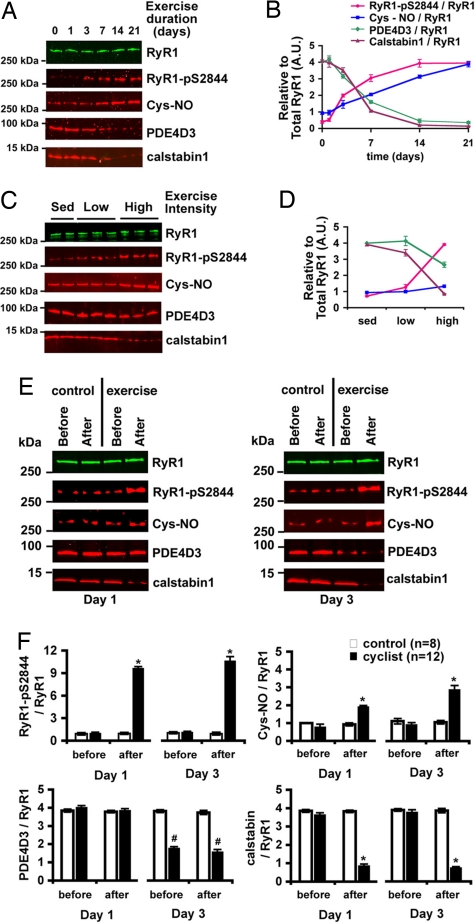
Fig. 1.
The RyR1 macromolecular complex undergoes remodeling during repeated exercise. (A) RyR1 complex in EDL muscle after exercise (twice daily swimming) analyzed by immunoprecipitation and immunoblotting for RyR1, RyR1-pS2844, cysteine S-nitrosylation of RyR1 (Cys-NO), and PDE4D3 and calstabin1 bound to the receptor. (B) Densitometric quantification of A, where each value is relative to the total RyR1 immunoprecipitated. RyR1 levels did not significantly change under any exercise condition. (C) Composition of the RyR1 complex in EDL muscle after low-intensity (swimming 15 min twice daily) and high-intensity (90 min twice daily) exercise for 5 days. (D) Densitometric quantification of C. (E) Immunoblot of the RyR1 complex immunoprecipitated from 100 μg of muscle homogenate from individual human thigh biopsies before and after exercise on days 1 and 3 of a cycling protocol (3 h at 70% VO2 max). Control cyclists sat in the exercise room but did not exercise. (F) Quantification by densitometry of E. Bar graphs depict PKA phosphorylation, S-nitrosylation, and PDE4D3, and calstabin1 levels in the RyR1 complex normalized to total RyR1 from control (n = 6) and exercise (n = 12) biopsies on each day. All data are mean ± SEM; *, P < 0.01 after exercise versus before; #, P < 0.01 exercise versus control. In all cases, the product of a single RyR1 immunoprecipitation was separated on a 4–20% gradient polyacrylamide gel, transferred, and probed for both total RyR1 and one or more of the modifications noted. The blots shown are representative of three or more independent experiments.