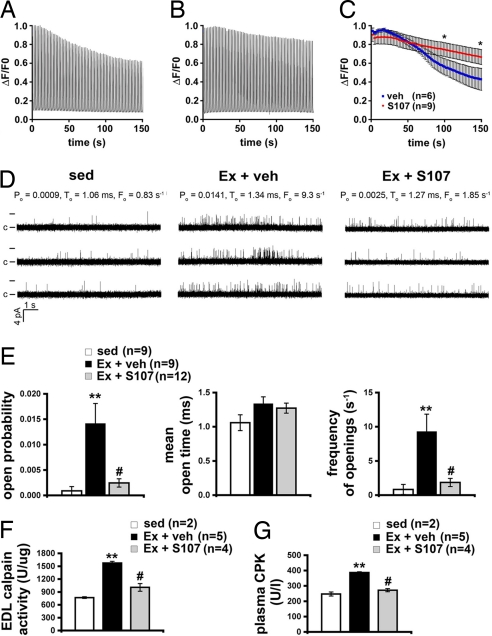
Fig. 4.
Stabilization of RyR1 channels slows muscle fatigue and reduces damage. (A) Representative trace of fluorescence (ΔF/F0) from a vehicle-treated FDB fiber loaded with fluo-4 normalized to the peak during repeated 300-ms, 120-Hz field-stimulated tetani at 0.5 Hz. Isolated cells were continuously perfused with Hepes-buffered Tyrodes solution at room temperature. (B) Representative (ΔF/F0) Ca2+ tetanic trace from an S107-treated FDB fiber. (C) Mean peak tetanic Ca2+ normalized to the peak during fatiguing stimulation (n = 6, vehicle; n = 9, S107). *, P < 0.05 unpaired t test. (D) Representative traces of RyR1 channel activity at 90 nM [Ca2+]cis from sedentary mice (sed, Left), mice exercised and treated with vehicle (Ex + veh, Center), and mice exercised and treated with S107 (Ex + S107, Right). Single channel openings are plotted as upward deflections; the open and closed (c) states of the channel are indicated by horizontal bars at the beginning of the traces. Channel open probability (Po), mean open time (To) and frequency of openings (Fo) are shown above each group of traces and represent average values from all experiments. (E) Average values of Po (Left), To (Center), and Fo (Right) of RyR1 from sedentary mice (sed, n = 9) and exercised mice treated either with vehicle (Ex + veh, n = 9) or S107 (Ex + S107, n = 12). (F) Calpain activity levels in EDL homogenates. (G) Plasma creatine kinase (CPK) activity levels in sedentary and exercised mice with, and without, calstabin1 rebinding with S107. Data are presented as mean ± SEM; **, P < 0.01 compared with sed; #, P < 0.01 compared with Ex + veh.