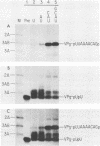
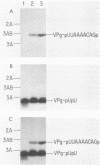
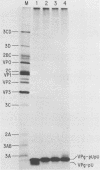
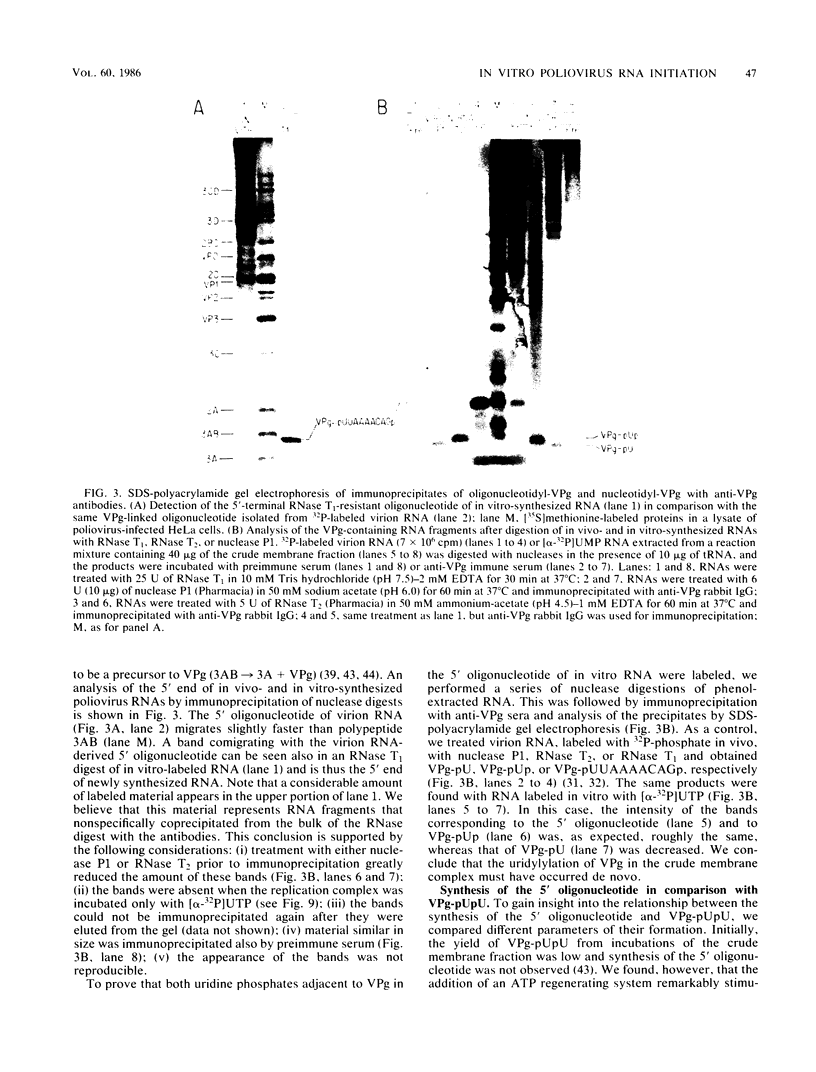
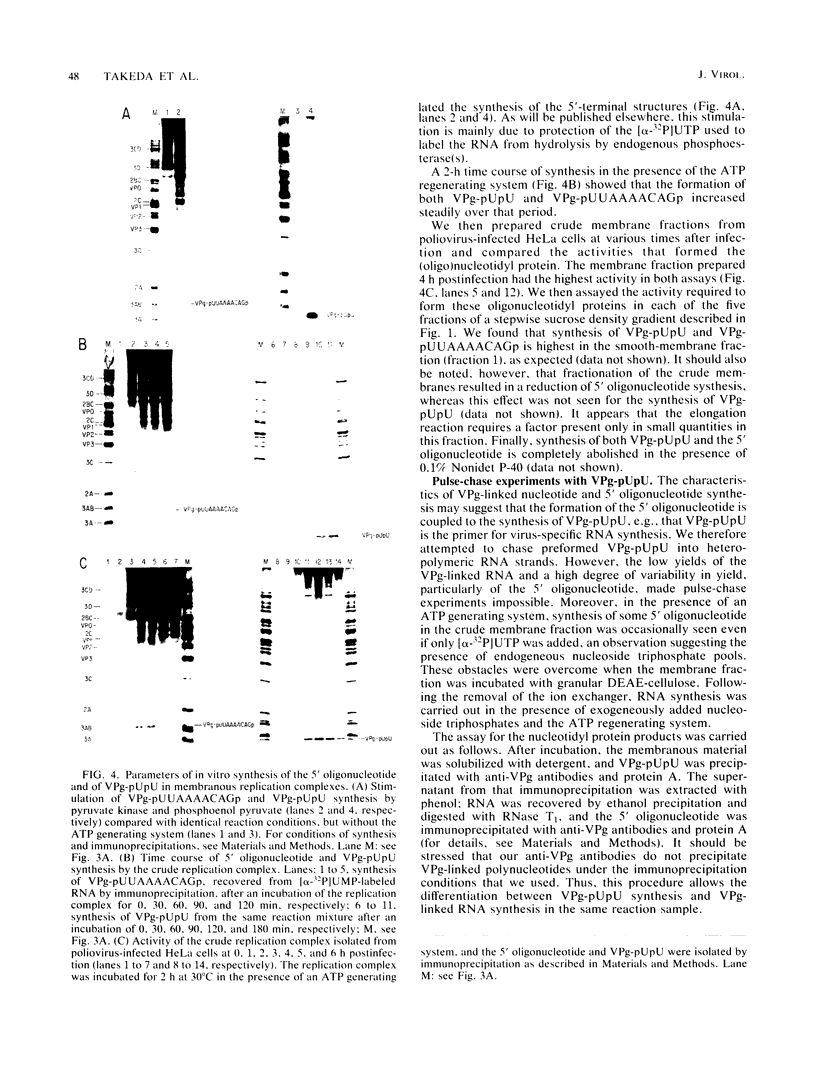
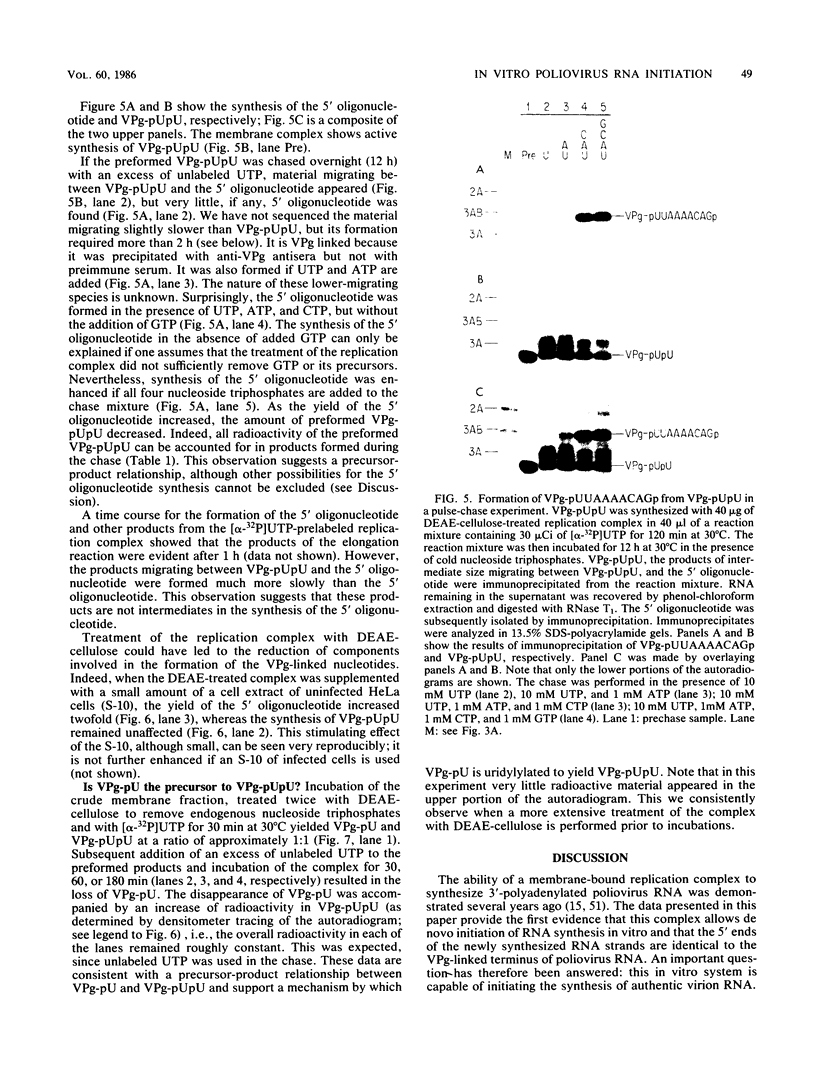
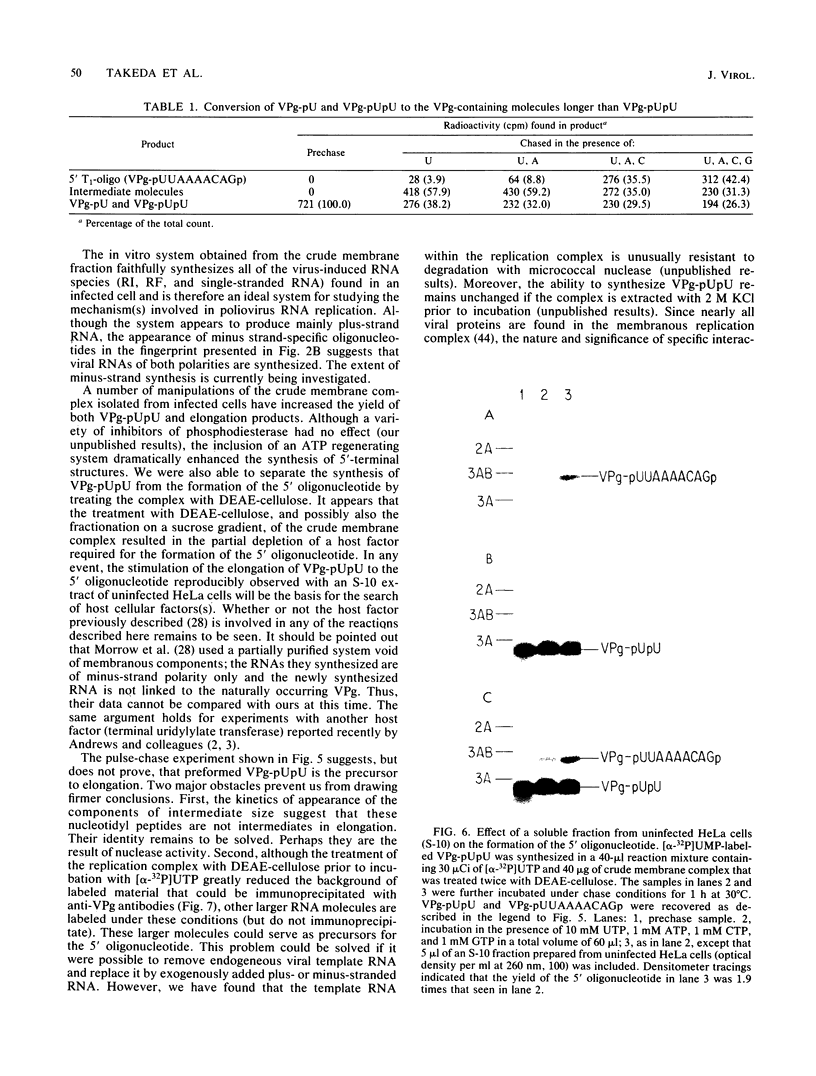
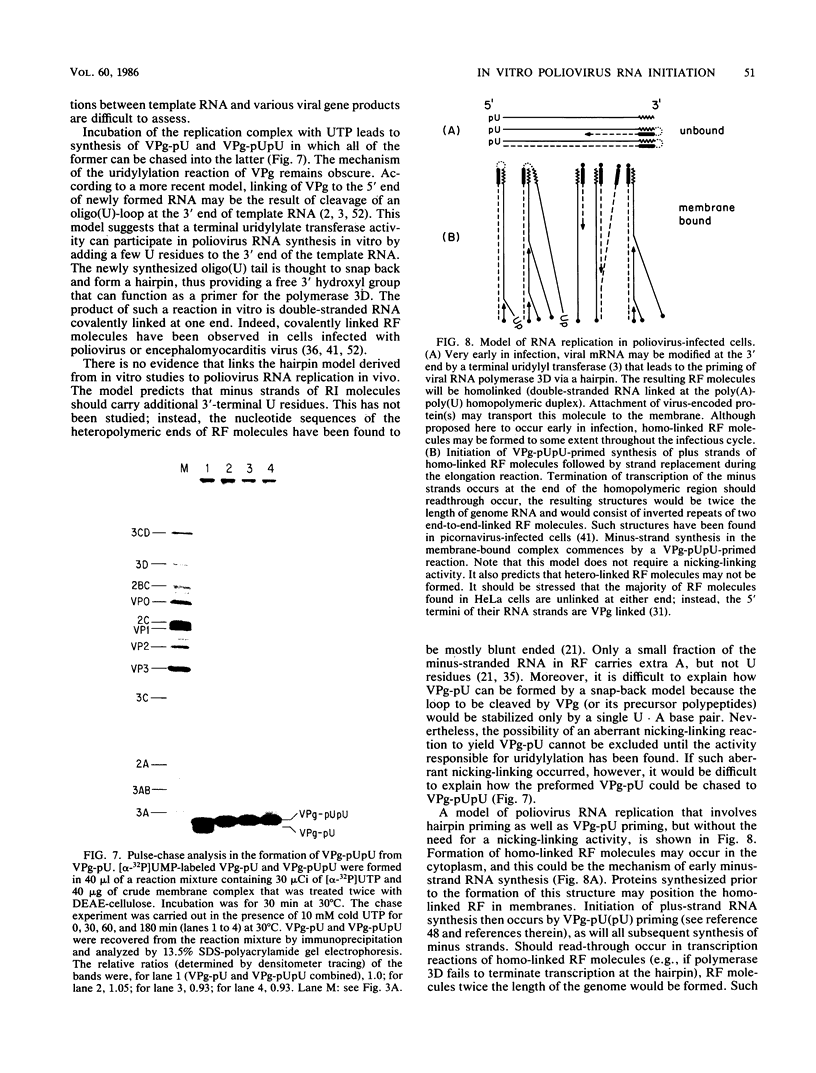
Abstract
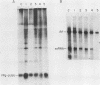
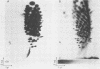
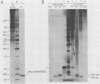
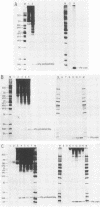
An in vitro poliovirus RNA-synthesizing system derived from a crude membrane fraction of infected HeLa cells was used to analyze the mechanism of initiation of poliovirus plus-strand RNA synthesis. This system contains an activity that synthesizes the nucleotidyl proteins VPg-pU and VPg-pUpU. These molecules represent the 5'-terminal structure of nascent RNA molecules and of virion RNA. The membranous replication complex is also capable of synthesizing nucleotidyl proteins containing nine or more of the poliovirus 5'-proximal nucleotides as assayed by the formation of the RNase T1-resistant oligonucleotide VPg-pUUAAAACAGp or by fingerprint analysis of the in vitro-synthesized RNA. Incubation of preformed VPg-pUpU with unlabeled nucleoside triphosphates resulted in the formation of VPg-pUUAAAACAGp. This reaction, which appeared to be an elongation of VPg-pUpU, was stimulated by the addition of a soluble fraction (S-10) obtained from uninfected HeLa cells. Preformed VPg-pU could be chased into VPg-pUpU in the presence of UTP. Our data are consistent with a model that VPg-pU can function as a primer for poliovirus plus-strand RNA synthesis in the membranous replication complex and that the elongation reaction may be stimulated by a host cellular factor.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambros V., Baltimore D. Protein is linked to the 5' end of poliovirus RNA by a phosphodiester linkage to tyrosine. J Biol Chem. 1978 Aug 10;253(15):5263–5266. [PubMed] [Google Scholar]
- Andrews N. C., Baltimore D. Purification of a terminal uridylyltransferase that acts as host factor in the in vitro poliovirus replicase reaction. Proc Natl Acad Sci U S A. 1986 Jan;83(2):221–225. doi: 10.1073/pnas.83.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews N. C., Levin D., Baltimore D. Poliovirus replicase stimulation by terminal uridylyl transferase. J Biol Chem. 1985 Jun 25;260(12):7628–7635. [PubMed] [Google Scholar]
- Baron M. H., Baltimore D. In vitro copying of viral positive strand RNA by poliovirus replicase. Characterization of the reaction and its products. J Biol Chem. 1982 Oct 25;257(20):12359–12366. [PubMed] [Google Scholar]
- Bienz K., Egger D., Rasser Y., Bossart W. Intracellular distribution of poliovirus proteins and the induction of virus-specific cytoplasmic structures. Virology. 1983 Nov;131(1):39–48. doi: 10.1016/0042-6822(83)90531-7. [DOI] [PubMed] [Google Scholar]
- Bienz K., Egger D., Rasser Y., Bossart W. Kinetics and location of poliovirus macromolecular synthesis in correlation to virus-induced cytopathology. Virology. 1980 Jan 30;100(2):390–399. doi: 10.1016/0042-6822(80)90530-9. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Compans R. W. The formation of poliovirus particles in association with the RNA replication complexes. J Gen Virol. 1973 Oct;21:99–108. doi: 10.1099/0022-1317-21-1-99. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. Characterization of poliovirus-specific structures associated with cytoplasmic membranes. Virology. 1970 Sep;42(1):112–122. doi: 10.1016/0042-6822(70)90243-6. [DOI] [PubMed] [Google Scholar]
- Caliguiri L. A., Tamm I. The role of cytoplasmic membranes in poliovirus biosynthesis. Virology. 1970 Sep;42(1):100–111. doi: 10.1016/0042-6822(70)90242-4. [DOI] [PubMed] [Google Scholar]
- Dasgupta A., Zabel P., Baltimore D. Dependence of the activity of the poliovirus replicase on the host cell protein. Cell. 1980 Feb;19(2):423–429. doi: 10.1016/0092-8674(80)90516-4. [DOI] [PubMed] [Google Scholar]
- Donis-Keller H., Maxam A. M., Gilbert W. Mapping adenines, guanines, and pyrimidines in RNA. Nucleic Acids Res. 1977 Aug;4(8):2527–2538. doi: 10.1093/nar/4.8.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donis-Keller H. Phy M: an RNase activity specific for U and A residues useful in RNA sequence analysis. Nucleic Acids Res. 1980 Jul 25;8(14):3133–3142. doi: 10.1093/nar/8.14.3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorsch-Häsler K., Yogo Y., Wimmer E. Replication of picornaviruses. I. Evidence from in vitro RNA synthesis that poly(A) of the poliovirus genome is genetically coded. J Virol. 1975 Dec;16(6):1512–1517. doi: 10.1128/jvi.16.6.1512-1517.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etchison D., Ehrenfeld E. Comparison of replication complexes synthesizing poliovirus RNA. Virology. 1981 May;111(1):33–46. doi: 10.1016/0042-6822(81)90651-6. [DOI] [PubMed] [Google Scholar]
- Flanegan J. B., Baltimore D. Poliovirus-specific primer-dependent RNA polymerase able to copy poly(A). Proc Natl Acad Sci U S A. 1977 Sep;74(9):3677–3680. doi: 10.1073/pnas.74.9.3677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanegan J. B., Petterson R. F., Ambros V., Hewlett N. J., Baltimore D. Covalent linkage of a protein to a defined nucleotide sequence at the 5'-terminus of virion and replicative intermediate RNAs of poliovirus. Proc Natl Acad Sci U S A. 1977 Mar;74(3):961–965. doi: 10.1073/pnas.74.3.961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanegan J. B., Van Dyke T. A. Isolation of a soluble and template-dependent poliovirus RNA polymerase that copies virion RNA in vitro. J Virol. 1979 Oct;32(1):155–161. doi: 10.1128/jvi.32.1.155-161.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanecak R., Semler B. L., Anderson C. W., Wimmer E. Proteolytic processing of poliovirus polypeptides: antibodies to polypeptide P3-7c inhibit cleavage at glutamine-glycine pairs. Proc Natl Acad Sci U S A. 1982 Jul;79(13):3973–3977. doi: 10.1073/pnas.79.13.3973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura N., Semler B. L., Rothberg P. G., Larsen G. R., Adler C. J., Dorner A. J., Emini E. A., Hanecak R., Lee J. J., van der Werf S. Primary structure, gene organization and polypeptide expression of poliovirus RNA. Nature. 1981 Jun 18;291(5816):547–553. doi: 10.1038/291547a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Larsen G. R., Dorner A. J., Harris T. J., Wimmer E. The structure of poliovirus replicative form. Nucleic Acids Res. 1980 Mar 25;8(6):1217–1229. doi: 10.1093/nar/8.6.1217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. F., Kitamura N., Nomoto A., Wimmer E. Sequence studies of poliovirus RNA. IV. Nucleotide sequence complexities of poliovirus type 1, type 2 and two type 1 defective interfering particles RNAs, and fingerprint of the poliovirus type 3 genome. J Gen Virol. 1979 Aug;44(2):311–322. doi: 10.1099/0022-1317-44-2-311. [DOI] [PubMed] [Google Scholar]
- Lee Y. F., Nomoto A., Detjen B. M., Wimmer E. A protein covalently linked to poliovirus genome RNA. Proc Natl Acad Sci U S A. 1977 Jan;74(1):59–63. doi: 10.1073/pnas.74.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y. F., Wimmer E. "Fingerprinting" high molecular weight RNA by two-dimensional gel electrophoresis: application to poliovirus RNA. Nucleic Acids Res. 1976 Jul;3(7):1647–1658. doi: 10.1093/nar/3.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxam A. M., Gilbert W. A new method for sequencing DNA. Proc Natl Acad Sci U S A. 1977 Feb;74(2):560–564. doi: 10.1073/pnas.74.2.560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonnell J. P., Levintow L. Kinetics of appearance of the products of poliovirus-induced RNA polymerase. Virology. 1970 Dec;42(4):999–1006. doi: 10.1016/0042-6822(70)90348-x. [DOI] [PubMed] [Google Scholar]
- Morrow C. D., Gibbons G. F., Dasgupta A. The host protein required for in vitro replication of poliovirus is a protein kinase that phosphorylates eukaryotic initiation factor-2. Cell. 1985 Apr;40(4):913–921. doi: 10.1016/0092-8674(85)90351-4. [DOI] [PubMed] [Google Scholar]
- Morrow C. D., Navab M., Peterson C., Hocko J., Dasgupta A. Antibody to poliovirus genome-linked protein (VPg) precipitates in vitro synthesized RNA attached to VPg-precursor polypeptide(s). Virus Res. 1984;1(2):89–100. doi: 10.1016/0168-1702(84)90066-2. [DOI] [PubMed] [Google Scholar]
- Mosser A. G., Caliguiri L. A., Scheid A. S., Tamm I. Chemical and enzymatic characteristics of cytoplasmic membranes of poliovirus-infected HeLa cells. Virology. 1972 Jan;47(1):30–38. doi: 10.1016/0042-6822(72)90235-8. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Detjen B., Pozzatti R., Wimmer E. The location of the polio genome protein in viral RNAs and its implication for RNA synthesis. Nature. 1977 Jul 21;268(5617):208–213. doi: 10.1038/268208a0. [DOI] [PubMed] [Google Scholar]
- Nomoto A., Kitamura N., Golini F., Wimmer E. The 5'-terminal structures of poliovirion RNA and poliovirus mRNA differ only in the genome-linked protein VPg. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5345–5349. doi: 10.1073/pnas.74.12.5345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedersen F. S., Haseltine W. A. A micromethod for detailed characterization of high molecular weight RNA. Methods Enzymol. 1980;65(1):680–687. doi: 10.1016/s0076-6879(80)65066-6. [DOI] [PubMed] [Google Scholar]
- Pettersson R. F., Ambros V., Baltimore D. Identification of a protein linked to nascent poliovirus RNA and to the polyuridylic acid of negative-strand RNA. J Virol. 1978 Aug;27(2):357–365. doi: 10.1128/jvi.27.2.357-365.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards O. C., Ehrenfeld E. Heterogeneity of the 3' end of minus-strand RNA in the poliovirus replicative form. J Virol. 1980 Nov;36(2):387–394. doi: 10.1128/jvi.36.2.387-394.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romanova L. I., Agol V. I. Interconversion of linear and circular forms of double-stranded RNA of encephalomyocarditis virus. Virology. 1979 Mar;93(2):574–577. doi: 10.1016/0042-6822(79)90260-5. [DOI] [PubMed] [Google Scholar]
- Rothberg P. G., Harris T. J., Nomoto A., Wimmer E. O4-(5'-uridylyl)tyrosine is the bond between the genome-linked protein and the RNA of poliovirus. Proc Natl Acad Sci U S A. 1978 Oct;75(10):4868–4872. doi: 10.1073/pnas.75.10.4868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueckert R. R., Wimmer E. Systematic nomenclature of picornavirus proteins. J Virol. 1984 Jun;50(3):957–959. doi: 10.1128/jvi.50.3.957-959.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semler B. L., Anderson C. W., Hanecak R., Dorner L. F., Wimmer E. A membrane-associated precursor to poliovirus VPg identified by immunoprecipitation with antibodies directed against a synthetic heptapeptide. Cell. 1982 Feb;28(2):405–412. doi: 10.1016/0092-8674(82)90358-0. [DOI] [PubMed] [Google Scholar]
- Semler B. L., Anderson C. W., Kitamura N., Rothberg P. G., Wishart W. L., Wimmer E. Poliovirus replication proteins: RNA sequence encoding P3-1b and the sites of proteolytic processing. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3464–3468. doi: 10.1073/pnas.78.6.3464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Senkevich T. G., Cumakov I. M., Lipskaya G. Y., Agol V. I. Palindrome-like dimers of double-stranded RNA of encephalomyocarditis virus. Virology. 1980 Apr 30;102(2):339–348. doi: 10.1016/0042-6822(80)90101-4. [DOI] [PubMed] [Google Scholar]
- Stanley J., Van Kammen A. Nucleotide sequences adjacent to the proteins covalently linked to the cowpea mosaic virus genome. Sequence determination after labelling in vitro using RNA ligase. Eur J Biochem. 1979 Nov 1;101(1):45–49. doi: 10.1111/j.1432-1033.1979.tb04214.x. [DOI] [PubMed] [Google Scholar]
- Takegami T., Kuhn R. J., Anderson C. W., Wimmer E. Membrane-dependent uridylylation of the genome-linked protein VPg of poliovirus. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7447–7451. doi: 10.1073/pnas.80.24.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takegami T., Semler B. L., Anderson C. W., Wimmer E. Membrane fractions active in poliovirus RNA replication contain VPg precursor polypeptides. Virology. 1983 Jul 15;128(1):33–47. doi: 10.1016/0042-6822(83)90316-1. [DOI] [PubMed] [Google Scholar]
- Tershak D. R. Association of poliovirus proteins with the endoplasmic reticulum. J Virol. 1984 Dec;52(3):777–783. doi: 10.1128/jvi.52.3.777-783.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Dyke T. A., Rickles R. J., Flanegan J. B. Genome-length copies of poliovirion RNA are synthesized in vitro by the poliovirus RNA-dependent RNA polymerase. J Biol Chem. 1982 Apr 25;257(8):4610–4617. [PubMed] [Google Scholar]
- Wimmer E. Genome-linked proteins of viruses. Cell. 1982 Feb;28(2):199–201. doi: 10.1016/0092-8674(82)90335-x. [DOI] [PubMed] [Google Scholar]
- Yin F. H., Knight E., Jr In vivo and in vitro synthesis of human rhinovirus type 2 ribonucleic acid. J Virol. 1972 Jul;10(1):93–98. doi: 10.1128/jvi.10.1.93-98.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yogo Y., Teng M. H., Wimmer E. Poly(U) in poliovirus minus RNA is 5'-terminal. Biochem Biophys Res Commun. 1974 Dec 23;61(4):1101–1109. doi: 10.1016/s0006-291x(74)80397-9. [DOI] [PubMed] [Google Scholar]
- Yogo Y., Wimmer E. Polyadenylic acid at the 3'-terminus of poliovirus RNA. Proc Natl Acad Sci U S A. 1972 Jul;69(7):1877–1882. doi: 10.1073/pnas.69.7.1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. C., Tuschall D. M., Flanegan J. B. Poliovirus RNA-dependent RNA polymerase and host cell protein synthesize product RNA twice the size of poliovirion RNA in vitro. J Virol. 1985 May;54(2):256–264. doi: 10.1128/jvi.54.2.256-264.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]