Abstract
Transcranial magnetic stimulation (TMS) is a method for focal brain stimulation based on the principle of electromagnetic induction, where small intracranial electric currents are generated by a powerful, rapidly changing extracranial magnetic field. Over the past 2 decades TMS has shown promise in the diagnosis, monitoring, and treatment of neurological and psychiatric disease in adults, but has been used on a more limited basis in children. We reviewed the literature to identify potential diagnostic and therapeutic applications of TMS in child neurology and also its safety in pediatrics. Although TMS has not been associated with any serious side effects in children and appears to be well tolerated, general safety guidelines should be established. The potential for applications of TMS in child neurology and psychiatry is significant. Given its excellent safety profile and possible therapeutic effect, this technique should develop as an important tool in pediatric neurology over the next decade.
Keywords: transcranial magnetic stimulation, corticospinal pathway maturation, cortical reorganization, corticospinal abnormalities, cortical excitability, cortical plasticity
Transcranial magnetic stimulation (TMS) is a 2-decade-old method for focal noninvasive brain stimulation that is based on the principles of electromagnetic induction, where small intracranial electrical currents are generated in the cerebral cortex by a powerful fluctuating extracranial magnetic field (see references 1 and 2 for a comprehensive review). In adult trials, TMS shows promise as a novel nonpharmacologic diagnostic and therapeutic technique with applications in neurology, psychiatry, and rehabilitation medicine. Yet, applications of TMS in the pediatric population have been slow to develop.
Preliminary success of this technique as a therapeutic tool for adult neurologic diseases such as stroke,3–5 major depression,6 and epilepsy7–9 should prompt similar development of this technique in the pediatric setting. Additionally, TMS can provide insight into normal and aberrant developmental neurology and neurophysiology in children. Recent reviews of the safety of TMS in adults and children suggest that there is minimal risk associated with this technique.10–12 The appendix lists all original studies that have included children in the study population. In this manuscript we review the current applications of TMS in the pediatric setting and provide a description of how TMS could be expanded to provide further benefit as a diagnostic and therapeutic technique in pediatrics.
Appendix.
Transcranial Magnetic Stimulation Studies Using Pediatric Participants
| Study Topic | Reference Numbers |
|---|---|
| Normal central pathway development | |
| Central motor conduction time | 25,27,28,32–36 |
| Motor threshold | 25–31 |
| Motor evoked potential latency | 28,36–38 |
| Motor evoked potential duration | 28 |
| Motor evoked potential amplitude | 27,28 |
| Intracortical inhibition | 30,31,41 |
| Silent period | 26,29,31,33,39,40 |
| Motor pathways reorganization | |
| Diplegia | 40,57 |
| Hemiplegia | 49–51,53–56 |
| Replantation | 58,59 |
| Neurological disorders | |
| Adrenoleukodystrophy | 66 |
| Alternating hemiplegia | 93 |
| Ataxia | 71–77 |
| Congenital mirror movements | 90,94,95 |
| Duchenne muscular dystrophy | 116 |
| Extrapyramidal disease | 82 |
| Hereditary motor and sensory neuropathy | 78,79 |
| Hereditary spastic paraplegia | 78 |
| Hirayama disease | 85 |
| Multiple sclerosis | 67 |
| Pelizaeus-Merzbacher | 80,87 |
| Primary lateral sclerosis | 81 |
| Rett syndrome | 96–99 |
| Spasticity | 88 |
| Spinal muscular atrophy II | 83 |
| Transverse myelitis | 68 |
| Wilson’s disease | 86 |
| Epilepsy | |
| Benign rolandic epilepsy | 102,109 |
| Generalized epilepsy | 106,112,113,115 |
| Myoclonic epilepsy | 105,110,111 |
| Partial epilepsy | 103,104, 106–108, 114,115 |
| Treatment | 138 |
| Psychiatric disorders | |
| Attention-deficit/hyperactivity disorder | 91,92,118–120,124 |
| Depression, schizophrenia | 132 |
| Tourette syndrome/tics | 118–121,123 |
| Medical disease | |
| Insulin-dependent diabetes | 84 |
| Malnutrition | 100,101 |
| Phenylketonuria | 117 |
| Scoliosis | 89 |
Prior to reviewing the pertinent literature, we provide a short primer on TMS. We describe the basic design of the TMS device. This is followed by a description of the 3 common stimulation techniques and relevant TMS-derived neurophysiological measurements. The review of the TMS studies in pediatrics is then divided into 3 sections focused on (1) diagnostic applications, (2) therapeutic application, and (3) TMS safety in children.
The TMS Technique
The 3 essential components of a TMS device are storage capacitors, a stimulating coil, and a timing mechanism.13,14 After being charged to approximately 5000 volts, the capacitors are discharged rapidly through a conductive coil over approximately 200 microseconds (Figure 1A).15 The current passing though the coil results in a fluctuating magnetic field which, in turn, induces a small intracranial electric current that focally stimulates the brain parenchyma. This is best evidenced by a motor response when the coil is placed over the primary motor cortex. Applied at appropriate intensity, stimulation of the primary motor cortex leads to gross movement and a measurable motor evoked potential in the hand contralateral to the stimulated cortex (Figure 1A).1 In this way, TMS represents a form of “electrode-less”, noninvasive cortical stimulation.16
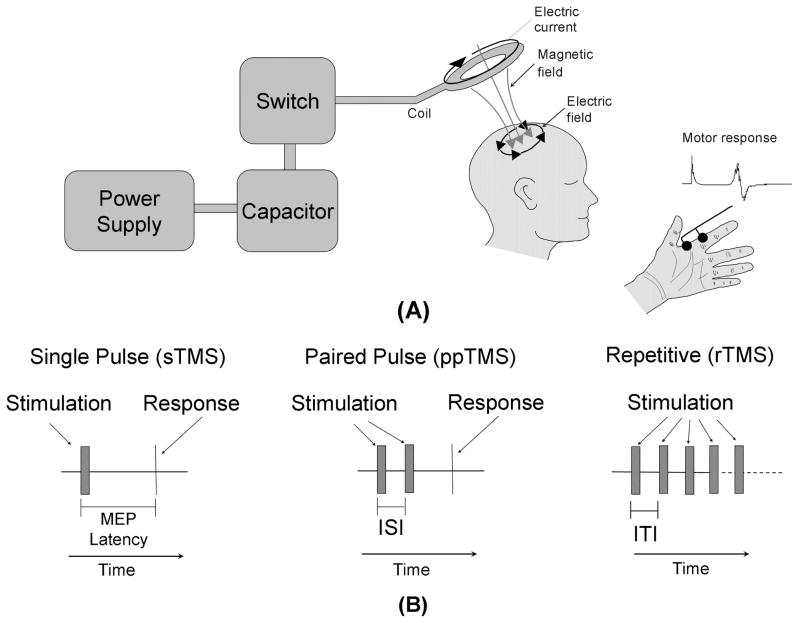
Figure 1.
TMS device and applications. (A) A block diagram depicting the transcranial magnetic stimulator (TMS) circuit is depicted on the left. The power supply charges the capacitor. An operator or computer then signals for the charge stored in the capacitor to be released into the stimulation coil through a thyristor switch. The current flowing through the stimulating coil (here depicted as a circular coil) produces a perpendicular magnetic field which transverses the skull and induces electrical currents within the cortex underlying the coil. A detectable muscle contraction, typically in a contralateral limb, results if the stimulation coil is placed over the motor cortex. This motor response is quantitatively measured as the motor evoked potential (MEP). (B) Three diagrams depicting various TMS protocols. Single-pulse TMS (sTMS) provides a single stimulation to the cortex. If the coil is positioned over the motor cortex, a motor response is elicited. Paired-pulse TMS (ppTMS) stimulates the motor cortex with 2 pulses separated by a short interstimulus interval (ISI). The resulting motor response is compared to the motor response elicited by signal-pulse TMS. Repetitive TMS (rTMS) uses a train of pulses separated by a regular intertrain interval (ITI) for a specific period of time. The duration of the intertrain interval determines the stimulation frequency.
Two types of TMS coils are typically used for human stimulation. A circular coil activates a large volume of brain tissue. A figure 8 coil is composed of 2 adjacent circular coils. The magnetic field of a figure 8 coil is focused at the point where the 2 circular coils meet, thereby providing stimulation to a focal area of brain tissue of approximately 1 cm3 in volume.
Three different TMS protocols are commonly used (Figure 1B)2:
sTMS. Single-pulse TMS stimulates the brain with one magnetic pulse. Single-pulse TMS is typically utilized to study corticospinal tract characteristics, but also can be used to interrupt ongoing cognitive activity, especially if the chronometry of such activity is known.
ppTMS. Paired-pulse TMS stimulates the motor cortex with 2 magnetic pulses that are separated by a variable inter-stimulus interval. The relative difference between the motor evoked potential amplitude following paired-pulse stimulation is compared to the motor evoked potential amplitude after single-pulse TMS. This ratio provides a measure of the cortical inhibitory-excitatory balance.
rTMS. Repetitive TMS stimulates the brain with a series of magnetic pulses. Notably, in most subjects repetitive TMS has an inhibitory effect on the cortex if the time between the pulses is 1 second or more (ie, 1 Hz or low-frequency repetitive TMS), and repetitive TMS has an excitatory effect on the cortex if the frequency of stimulation is faster than 10 Hz and applied in bursts of 2 to 10 seconds with pauses of 20 to 30 seconds in between (ie, high-frequency repetitive TMS).2
The 3 aforementioned TMS techniques are commonly used to study 3 categories of neurophysiological measurements: (1) cortical excitability, (2) corticospinal pathway characteristics, and (3) interhemispheric dynamics.
Cortical excitability. This can be measured with 3 neurophysiological measurements: motor threshold, silent period, and the intracortical inhibitory-excitatory balance. Motor threshold is defined by the lowest strength single-pulse TMS pulse required to produce a criterion amplitude motor evoked potential (eg, 5 μV peak-to-peak) on a prespecified fraction of trials (eg, 5 out of 10) with optimal placement of the coil over the contralateral motor cortex. Motor threshold is typically reported as a percentage of the maximum stimulator output rather than the absolute magnetic field strength. However, it should be noted that different stimulation devices may have different output strengths. Next, the silent period is the brief suppression of voluntary contraction in a contralateral target muscle following a single cortical stimulus to the motor cortex. The silent period includes cortical and spinal mechanisms, and appears to be supported in part by mechanisms independent of those generating the motor evoked potential that rely prominently on GABA-B neurotransmitter activity. Finally, intracortical inhibitory-excitatory balance is determined using paired-pulse TMS. The motor evoked potential amplitude elicited by a stimulation pulse that is preceded by a conditioning pulse is determined with various interstimulus intervals ranging from 1 to 500 milliseconds. Short interstimulus intervals result in intracortical inhibition, which is presumably mediated by GABA, and longer interstimulus intervals result in intracortical facilitation, which is presumably mediated primarily by glutamate.
Corticospinal pathway characteristics. These are determined by examining several neurophysiological measurements derived from the motor evoked potential, such as amplitude, duration, and onset latency. Since the motor evoked potential latency provides an index of the combined peripheral and central motor pathway latency, the peripheral pathway latency must be subtracted from the motor evoked potential latency in order to derive the central motor conduction time–a measure of corticospinal (ie, central nervous system) neural transmission speed. Peripheral pathway latency is estimated by either stimulating the proximal segment of the appropriate spinal roots by placing the TMS coil at the relevant spinal level or (more accurately) from the F-wave latency.
Interhemispheric dynamics. This can be measured by determining the ipsilateral silent period. This measurement uses single-pulse TMS to stimulate inhibitory transcallosal connections from the ipsilateral to the contralateral hemisphere, which, in turn, interrupts ongoing ipsilateral electromyographic activity.17 Alternatively, paired-pulse TMS can be used by applying one stimulus to one hemisphere with the second one at a variable interval to the other. The first stimulus results in a response in contralateral muscles, but also conditions, via callosal fibers, the response to the second stimulus. The interstimulus interval provides a measure of callosal conduction time.
Diagnostic Applications of TMS in Children
TMS has been used to study developmental neurophysiology and neurophysiologic abnormalities that result from neurological, psychiatric, and medical disease. This section is divided into 3 topics. First, normal developmental neurophysiology of the corticospinal motor tract as defined by TMS studies is reviewed. This information can help define basic developmental neurophysiological mechanisms and is the beginning of the development of a normative database of neurophysiological measurements that could be used as a diagnostic reference in the clinical setting. Second, we review studies that use TMS to investigate cortical motor map reorganization following congenital or postnatal central nervous system injury and following peripheral nerve injury as the result of limb amputation. Last, we review studies that have used TMS to investigate neurophysiological abnormalities in children with neurological, psychiatric, and medical diseases.
Motor Pathway Maturation in Neurologically Normal Children
The quality and speed of motor movements are known to improve during childhood.18,19 Poor quality and slow motor movements are associated with neurodevelopmental abnormalities as a consequence of such disorders as premature birth, learning disabilities, and neurobehavioral disorders. Abnormalities in the quality and speed of motor movements are measured clinically by “soft neurological” signs. However, the developmental trajectory of these “soft neurological” signs is variable, resulting in a significant overlap between children with normal development and those at risk for abnormal development.18,19 The ability to measure motor maturation is important in light of the fact that abnormal early motor pathway maturation may be associated with impaired cognitive development later in childhood20–23 and adulthood.24 As described below, TMS may provide a way in which motor maturation can be objectively and accurately measured and followed during childhood.
Objective measures of motor development as indexed by TMS measures could indeed define the significance of clinical correlates of motor development, such as “soft neurological” signs, and further define the normal trajectory of motor development. TMS studies have provided insight into normal neurophysiological changes that occur during motor system maturation, particularly with respect to the development of crossed and uncrossed corticospinal motor pathways. Table 1 outlines these studies and their findings. All studies listed in Table 1 have examined developmental changes in at least one neurophysiological measurement. The protocol used in each study is listed under each heading. Crossed and uncrossed refer to whether the motor evoked potential was recorded from the limb contralateral or ipsilateral to the hemisphere stimulated with the TMS coil, respectively. Relaxed and facilitated refer to whether the participants were required to actively contract the target muscle during TMS stimulation. The results are divided into 3 categories depending on whether there was a decrease, increase, or no change in the neurophysiological measurement value with age (as indicated with “From age to age”) or whether children demonstrated a different neurophysiological measurement value as compared to adults (as indicated with “age to age vs adult”).
Table 1.
Developmental Changes by Age in TMS-Derived Neurophysiological Measurements
| Decrease | No Change | Increase | |
|---|---|---|---|
| Motor Threshold | |||
| Crossed, Relaxed | |||
| Masur et al (1995)26 | From 3 to 14 y | ||
| Muller et al (1991)27 | From 2 wk to 10 y | ||
| Nezu et al (1997)28 | From 1 y to adult | ||
| Moll et al (1999)31 | From 8 to 16 y | ||
| Crossed, Facilitated | |||
| Eyre et al (2001)25 | From 1 to 16 y | From neo to 3 mo | |
| Heinen et al (1998)29 | 4 to 7 y vs adult | ||
| Mall et al (2004)30 | From 6 y to adult | ||
| Masur et al (1995)26 | From 3 to 14 y | ||
| Moll et al (1999)31 | From 8 to 16 y | ||
| Uncrossed, Facilitated | |||
| Eyre et al (2001)25 | From 1 to 16 y | From neo to 3 mo | |
| Central Motor Conduction Time | |||
| Crossed, Relaxed | |||
| Fietzek et al (2000)32 | From 2 mo to 10 y | From 10 y to 40 y | |
| Heinen et al (1998)33 | 6 to 9 y vs adult | ||
| Nezu et al (1997)28 | From 1 to 12 y | 12 to 14 y vs adult | |
| Muller et al (1992)35 | From 2 to 13 ya | ||
| Muller et al (1991)27 | From 2 wk to 10 y | ||
| Muller et al (1991)27 | From 2 wk to 13 ya | ||
| Crossed, Facilitated | |||
| Eyre et al (2001)25 | From neo to 15 mo | From 15 mo to 16 y | |
| Fietzek et al (2000)32 | From 2 mo to 5 y | From 5 to 40 y | |
| Heinen et al (1998)33 | 6 to 9 y vs adult | ||
| Muller et al (1997)36 | 3 to 11 y vs adult | ||
| Nezu et al (1999)34 | From 2 to 10 y | 10 to 13 y vs adult | |
| Uncrossed, Facilitated | |||
| Eyre et al (2001)25 | From neo to 16 y | ||
| Motor Evoked Potential Latency | |||
| Crossed, Relaxed | |||
| Caramia et al (1993)37 | From 2 to 12 y | ||
| Nezu et al (1997)28 | From 1 y to adult | ||
| Crossed, Facilitated | |||
| Caramia et al (1993)37 | From 2 to 12 y | ||
| Koh and Eyre (1988)38 | From preme to 8 y | From preme to 8 ya | |
| Uncrossed, Facilitated | |||
| Muller et al (1997)36 | Absent after 10 y | Longer than crossed until 10 \ | |
| Motor Evoked Potential Duration | |||
| Crossed, Relaxed | |||
| Nezu et al (1997)28 | From 1 y to adult | ||
| Motor Evoked Potential Amplitude | |||
| Crossed, Relaxed | |||
| Muller et al (1991)27 | From 2 wk to 10 y | ||
| Nezu et al (1997)28 | From 1 to 10 y | From 10 y to adult | |
| Silent Period | |||
| Contralateral, Latency | |||
| Heinen et al (1998)33 | 6 to 9 y vs adult | ||
| Masur et al (1995)26 | From 3 to 14 y | ||
| Contralateral, Duration | |||
| Heinen et al (1998)33 | 4 to 6 y vs adult | ||
| Moll et al (1999)31 | From 8 to 16 y | ||
| Ipsilateral, Latency | |||
| Garvey et al (2003)39 | From 6 y to adult | ||
| Heinen et al (1998)29 | 4 to 6 y vs adult | ||
| Heinen et al (1999)40 | 10 to 15 y vs adult | ||
| Masur et al (1995)26 | From 3 to 14 y | ||
| Ipsilateral, Duration | |||
| Garvey et al (2003)39 | From 6 y to adult | ||
| Intracortical Inhibition | |||
| Bender et al (2005)41 | From 6 to 10 y | ||
| Mall et al (2004)30 | From 6 y to adult | ||
| Moll et al (1999)31 | From 8 to 16 y | ||
NOTE: neo = neonate; preme = premature neonate.
Certain studies have corrected parameters for height of the participant.
Crossed Corticospinal Pathway Development
TMS studies suggest that the crossed corticospinal pathways develop throughout childhood. Motor threshold appears to increase over the first 3 months of life25 but then linearly decreases until adolescence, with the adult motor threshold reached in early adolescence.25–31 This relationship has been confirmed in the upper and lower extremities27 with muscles in both relaxed and facilitated state.26 Crossed central motor conduction time when the target muscle is relaxed clearly decreases during childhood.25,27,28,32–36 Such developmental changes are found to continue into early adolescence if participant height is taken into account in the calculation of crossed central motor conduction time.27,35 If the study protocol uses motor facilitation, the developmental changes in crossed central motor conduction time end in early childhood.25,32–34,36 Crossed central motor conduction times in both distal upper and lower extremities appear to have similar developmental patterns27 but may be different in proximal as compared to distal muscles.34 Motor evoked potential latency may decrease or increase during childhood depending on whether the stimulation protocol uses facilitation37 or if this parameter is corrected for participant height.38 Motor evoked potential amplitude has been reported to increase with age.27,28
Uncrossed Corticospinal Pathway Development
Uncrossed corticospinal pathways may be extremely important during recovery from brain injury. These pathways may be clinically represented by developmental mirror movements and neurophysiologically represented by motor evoked potentials ipsilateral to the hemisphere stimulated with single-pulse TMS. One TMS study suggests that the uncrossed corticospinal pathway is faster than the normally predominant crossed corticospinal pathway before 6 months of age.25 Another study detected the uncrossed corticospinal pathways in most children before 10 years of age and found it to be more prevalent in proximal as compared to distal muscles.36
Development of Cortico-Cortical Connections
Cortico-cortical connections also undergo developmental change. Such change may account for the maturation of motor task performance that continues beyond childhood.32,33 Cortico-cortical and interhemispheric inhibition, as measured by the ipsilateral silent period, may be minimal at or before 5 years of age,29 but increases with age,39 reaching adult equivalent values in adolescence.40 However, not all studies have been able to document cortico-cortical inhibition in childhood and early adolescence.26 Developmental changes in the contralateral silent period duration,29,31 but not latency,26,33 have also been detected.
Intracortical Pathway Development
Developmental changes in intracortical inhibition have been measured using several protocols. The paired-pulse TMS method has been used to demonstrate a developmental increase in intracortical inhibition in one study30 and no change in intracortical inhibition or facilitation in another study.31 Other studies have examined the developmental changes in a TMS-evoked electrophysiological cortical response that may represent inhibitory processes.41
Future Studies
Further studies must account for factors that result in measurement variation, such as participant height,27 peripheral pathway latency,27 amount of baseline muscle contraction,37 and skull and brain size.42,43 Given the many dynamic factors that occur during neural system maturation, such as synaptic pruning and development,25 myelination of the corticospinal, intracortical, and transcortical pathways,44 changes in axonal diameter and length,45 and organization of pyramidal neuron-firing patterns,46 the influence of developmental factors on corticospinal maturation may be better defined if future studies combine TMS with anatomic and functional measures of corticospinal pathway organization. In the future, anatomical neuroimaging will be essential for minimizing factors that contribute to measurement variation. For example, differences in skull and scalp thickness and cerebral spinal fluid volume that can critically influence the distribution of the induced intracerebral current could be taken into account.47,48 Clearly combining anatomic and functional information will help define normal development. Such information will greatly assist in developing normative developmental trajectories that may be useful for the early detection of developmental neurological abnormalities.
Cortical Reorganization
Central Injury
Although it is believed that early brain injury, in general, is associated with better recovery than brain injury later in childhood, recovery from early brain injury is still very variable, and the mechanisms of recovery are still poorly understood. Ipsilateral corticospinal projections are believed to play a significant role in recovery from lesions affecting the motor system. As briefly discussed above, ipsilateral corticospinal projections may be prominent very early in life and can be neurophysiologically detected into adolescence.25 The exact pathway through which ipsilateral corticospinal neurons project to the affected limb following recovery from brain injury is not well understood. Normal ipsilateral corticospinal, corticoreticulospinal, or corticopropriospinal tract projections could play a role during recovery from unilateral brain injury. However, ipsilateral fetal corticospinal projections that normally regress at, or before, infancy could persist and aid in recovery. In addition, at the spinal level, contralateral axons could sprout terminal connections to the α-motorneurons of muscles in the affected limb.
In order to characterize the corticospinal projections from the ipsilateral hemisphere, investigators have compared motor evoked potential characteristics from analogous ipsilateral and contralateral muscles during single-pulse TMS. Some investigators have compared the ipsilateral cortical silent period durations and latencies,49 or motor evoked potential amplitude, duration, and latency25,50–52 in combination with, or instead of, cross-correlation analysis.50,51,53,54 The distribution and overlap of ipsilateral and contralateral cortical motor maps in the ipsilateral hemisphere have also been studied.55 In addition, single-pulse TMS has been combined with functional neuroimaging to clarify cortical activation patterns elicited by motor movement in individuals with congenital, perinatal, or neonatal unilateral brain injury.52,56
A review of the TMS studies evaluating children with developmental and acquired hemiplegia and diplegia reveals several patterns of neural reorganization. Overall, TMS studies demonstrate at least 4 neurophysiological patterns of cortical reorganization in children with hemiplegia and at least 2 neurophysiological patterns of cortical reorganization in children with diplegia.
Hemiplegia
Following unilateral brain injury, 4 neurophysiological patterns of neural reorganization can be defined by whether application of single-pulse TMS to one, both, or neither hemisphere can evoke a motor evoked potential in the affected limb. These patterns may correlate with age and severity of the injury (see Figure 2). For example, unilateral brain injury after 2 years of age, when severe, may be associated with an inability to elicit a motor evoked potential in the affected limb by application of single-pulse TMS to either hemisphere. This suggests that no functional corticospinal pathways project to the affected limb from either hemisphere and probably indicates a poor recovery potential.50,51 This pattern of recovery does not appear if the injury occurs in or before infancy. Many children with injury in or before infancy were found to have unilateral lateralization of the motor system with the corticospinal pathway originating from the hemisphere ipsilateral to the affected limb. In these children, a motor evoked potential in the affected limb could be elicited only with application of single-pulse TMS to the ipsilateral hemisphere. This pattern appeared to be more common in children with hemiplegia acquired before birth (ie, congenital hemiplegia or hemiplegia associated with cortical dysplasia or perinatal hypoxia).50,51,53,54 For a minority of children with hemiplegia acquired during the perinatal period and a few children with hemiplegia acquired after the neonatal period, a motor evoked potential in the affected limb could be elicited by application of single-pulse TMS to either hemisphere, suggesting bilateral corticospinal pathway projections to the affected limb.50,51,56 However, for many children with more mild hemiplegia, especially those who acquired hemiplegia in or after the infantile period, a motor evoked potential in the affected limb could be elicited only by application of single-pulse TMS to the contralateral hemisphere, suggesting that the corticospinal pathway found in normal children predominates.49
Figure 2.
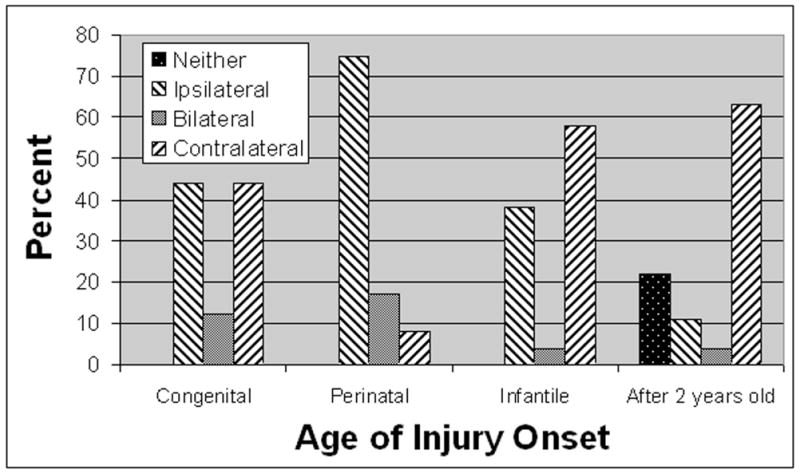
Reorganization patterns following recovery in acquired hemiplegia organized by age at which the cortical damage occurred. Note that the ipsilateral hemisphere is more often involved in motor control of the affected limb before 2 years of age, suggesting a greater preservation of the capacity for successful motor remapping for brain injury occurring early in life. The hemisphere of origin of the corticospinal projections to the affected limbs as determined by single-pulse TMS is shown. This figure was produced by reviewing all available studies in which the results of single-pulse TMS stimulation for individual cases of acquired hemiplegia were available.
The patterns described above are based on motor evoked potentials recorded from distal upper extremity muscles. Slightly different patterns of reorganization have been reported for proximal upper extremity muscles.55 These impressive preliminary findings suggest that further studies have an excellent opportunity to define patterns of reorganization in congenital and early acquired hemiplegia. The limited sample sizes in many of these studies restrict the ability to generalize these findings.
Diplegia
Two neurophysiological patterns of neural reorganization in spastic diplegia can be defined by whether application of single-pulse TMS to either hemisphere elicits a motor evoked potential in both limbs or in only one limb. For example, a motor evoked potential could be elicited in either limb with application of single-pulse TMS to either hemisphere for children with symmetric diplegia.57 This suggests that both hemispheres reorganized to provide ipsilateral and contralateral corticospinal projections (or never organized to project predominantly contralateral corticospinal projections). However, single-pulse TMS to either hemisphere could elicit a motor evoked potential in the more affected, but not the less affected, limb in children with asymmetric diplegia.57 A motor evoked potential in the less affected limb could be elicited only with single-pulse TMS applied to the contralateral hemisphere. This suggests that the less damaged hemisphere reorganized to provide motor output to both the ipsilateral and contralateral limbs, while the more damaged hemisphere only provided projections to the more affected limb. Thus, it appears that reorganization in spastic diplegia may be dependent on the symmetry of the insult. Reorganization may also be dependent on prematurity. Motor maps of the optimal single-pulse TMS scalp site for eliciting a motor evoked potential in the lower extremities appeared to be significantly shifted laterally in premature, but not term, children with diplegia.57 One study found that transcallosal inhibition may be absent in adolescents with diplegic cerebral palsy.40
Peripheral Nerve Injury
Single-pulse TMS has revealed cortical motor remapping as a consequence of amputation and replantation. The motor map of the contralateral deltoid was shown to expand after arm amputation without replant.58 In another study, the motor maps of the first dorsal interosseous were unchanged following hand replantation, but hand function was limited, and the bicep brachii motor map was larger and shifted toward the motor map of the replanted hand.59
Future Studies
Although TMS is clearly useful for revealing changes in motor maps and corticospinal pathway reorganization following recovery from brain injury, the significance of the various patterns of reorganization and the particular association between these patterns and the etiology of the brain damage is not clear. Many studies contain small sample sizes or mixed etiologies (ie, genetic, congenital, and acquired brain lesions) or lesion type (ie, cortical and subcortical). In future studies, well-selected homogenous populations may help link patterns of reorganization with etiology. In addition, monitoring dynamic neurophysiological changes during recovery will be essential. For example, acute neurophysiological changes in the motor evoked potential60–64 and subacute changes in the motor map65 that follow stroke have prognostic correlation with ultimate functional recovery in adults.
Studies using TMS in children have uncovered important information regarding CNS reorganization following central and peripheral nervous system injury. In the future, studying well-defined populations with TMS combined with other neuroimaging tools will provide insight into the neuroplasticity involved in successful and suboptimal recovery from brain damage. As TMS becomes more widely utilized by adult and child neurologists, this tool may become essential for the prognostic assessment in congenital and acquired brain injury.
Diagnostic Application in Neurological Disease
TMS has been used to demonstrate neurophysiological changes as a consequence of neurologic and medical disorders of childhood. TMS is particularly useful for interrogating the integrity of the corticospinal tract and measuring cortical excitability. These studies foreshadow the utility of TMS in the diagnosis and management of neurological disorders in children. Table 2 outlines the abnormalities in common TMS-derived neurophysiological measurements for specific neurologic and medical disorders. Seven common TMS-derived neurophysiological measurements are presented in this table. Certain studies may have considered other neurophysiological measurements not specifically represented in this table. Symbols represent changes in the specific neurophysiological measurement value as compared to values derived from control participants or the normal population (see legend at bottom of table).
Table 2.
Changes in TMS-Derived Neurophysiological Measurements in Disorders of Childhood
| MT | CMCT | MEP Latency | MEP Amp | SP | ICI | ICF | |
|---|---|---|---|---|---|---|---|
| Neurologic Disorders | |||||||
| Adrenoleukodystrophy | |||||||
| Nezu et al (1996)66 | ↔ | ↔ | |||||
| Autosomal dominant cerebellar ataxia | |||||||
| Lanzillo et al (1994)74 | ↔ | ↓ | |||||
| Childhood onset altering hemiplegia | |||||||
| Nezu et al (1997)93 | ↑ | ↓ | |||||
| Childhood onset hereditary extrapyramidal disease | |||||||
| Muller et al (1992)82 | ↔ | ↔ | |||||
| Congenital mirror movements | |||||||
| Cincotta et al (1996)95 | ↓ | ||||||
| Maegaki et al (2002)90 | ↑ | ||||||
| Reitz et al (1998)94 | ↑ | ||||||
| Duchenne muscular dystrophy | |||||||
| Di Lazzaro et al (1998)116 | ↑ | ↔ | |||||
| Early onset non-Friedreich’s ataxia | |||||||
| Mondelli et al (1995)75 | ↑/↔ | ||||||
| Early onset recessive ataxia | |||||||
| Lanzillo et al (1994)74 | ↑ | ↓ | |||||
| Familial childhood primary lateral sclerosis | |||||||
| Gascon et al (1995)81 | ↔ | ≠ | ≠ | ||||
| Friedreich’s ataxia | |||||||
| Claus et al (1988)71 | ↑ | ||||||
| Cruz Martinez et al (1992)72 | ↑ | ↑ | ↓ | ||||
| Cruz Martinez et al (1997)73 | ↑ | ↑ | ↓ | ||||
| Lanzillo et al (1994)74 | ↑ | ↓ | |||||
| Mondelli et al (1995)75 | ↑ | ↓ | |||||
| Santoro et al (2000)76 | ↑ | ||||||
| Schwenkreis et al (2002)77 | ↔ | ↑ | ↔ | ||||
| Hereditary motor and sensory neuropathy I & II without associated pyramidal features | |||||||
| Claus et al (1990)78 | ↔ | ||||||
| Hereditary motor and sensory neuropathy I with associated pyramidal features | |||||||
| Claus et al (1990)78 | ↑ | ||||||
| Hereditary motor and sensory neuropathy II with associated pyramidal features | |||||||
| Claus et al (1990)78 | ↑/↔ | ||||||
| Hereditary spastic paraplegia | |||||||
| Claus et al (1990)78 | ↔ | ||||||
| Hirayama disease | |||||||
| Shizukawa et al (1994)85 | ↑ | ||||||
| Infantile onset Pelizaeus-Merzbacher | |||||||
| Nezu et al (1996,1998)80,87 | ≠ | ≠ | |||||
| Multiple sclerosis | |||||||
| Dan et al (2000)67 | ↓ | ↑ | |||||
| Rett syndrome | |||||||
| Eyre et al (1990)96 | ↓ | ↓ | |||||
| Heinen and Korinthenberg (1996)97 | ↓ | ||||||
| Heinen et al (1997)98 | ↓ | ||||||
| Nezu et al (1998)99 | ↓ | ↓ | ↔ | ||||
| Spasticity | |||||||
| Dachy and Dan (2002)88 | ↑ | ||||||
| Spinal muscular atrophy type II | |||||||
| Imai et al (1995)83 | ↔ | ↓ | |||||
| Transverse myelitis | |||||||
| Noguchi et al (2000)68 | ↑ | ↓ | |||||
| Wilson’s disease | |||||||
| Meyer et al (1991)86 | ↑ | ↓ | |||||
| Epilepsy | |||||||
| Benign rolandic epilepsy | |||||||
| Manganotti and Zanette (2000)109 | ↑ | ||||||
| Nezu et al (1997)102 | ↑ | ↔ | ↔ | ||||
| Generalized epilepsy | |||||||
| Brodtmann et al (1999)112 | ↑ | ||||||
| Ertas et al (2000)115 | ↑ | ||||||
| Macdonell et al (2001) 113 | ↔ | ↑ | |||||
| Tataroglu et al (2004)106 | ↑ | ||||||
| Myoclonic epilepsy | |||||||
| Manganotti et al (2001)110 | ↓ | ||||||
| Reutens et al (1993)105 | ↑ | ↔ | ↔ | ||||
| Valzania et al (1999)111 | ↔ | ↔ | ↔ | ↓ | ↑ | ||
| Partial epilepsy | |||||||
| Cincotta et al (1998)114 | ↑/↔ | ||||||
| Cantello et al (2000)104 | ↑ | ↔ | ↓ | ↑ | |||
| Ertas et al (2000)115 | ↑ | ||||||
| Inghilleri et al (1998)107 | ↑ | ↓ | ↓ | ||||
| Michelucci et al (1996)103 | ↑ | ↔ | ↔ | ↔ | |||
| Shimizu et al (2001)108 | ↑ | ↑ | |||||
| Tataroglu et al (2004)106 | ↑ | ↑ | |||||
| Psychiatric Disorders | |||||||
| Attention-deficit/hyperactiviy disorder | |||||||
| Garvey et al (2005)124 | ↔ | ↓ | |||||
| Moll et al (2000)120 | ↔ | ↔ | ↓,↑a | ↔ | |||
| Moll et al (2001)118 | ↔ | ↓,↑a | ↔ | ||||
| Ucles et al (1996)91 | R>L | ||||||
| Ucles et al (2000)92 | ↑ | ||||||
| Attention-deficit/hyperactiviy disorder and Tourette syndrome/tics | |||||||
| Moll et al (2001)119 | ↔ | ↓ | ↓ | ||||
| Moll et al (2001)118 | ↓ | ↓ | |||||
| Tourette syndrome/tics | |||||||
| Moll et al (2001)118 | ↔ | ↓ | ↔ | ↔ | |||
| Moll et al (1999)123 | ↔ | ↓ | ↔ | ↔ | |||
| Ziemann et al (1997)121 | ↔ | ↓b | ↓b | ||||
| Medical disorders | |||||||
| Insulin-dependent diabetes | |||||||
| D’Annunzio et al (1995)84 | ↑ | ↓ | |||||
| Malnutrition | |||||||
| Karak et al (1999)100 | ↑ | ↑ | ↓ | ||||
| Tamer et al (1997)101 | ↑ | ↑ | |||||
| Phenylketonuria | |||||||
| Roricht et al (1999)117 | ↑ | ↓ | ↓ | ||||
| Scoliosis | |||||||
| Tabaraud et al (1993)89 | ↔ | ||||||
NOTE: MT = motor threshold; CMCT = central motor conduction time; MEP = motor evoked potential; SP = silent period; ICI = intracortical inhibition; ICF = intracortical facilitation.
Symbols: ↔ no difference in the parameter value between normal and clinical group; ↑ a higher parameter value in the clinical group as compared to normal group; ↓ a lower parameter value in the clinical group as compared to normal group; ≠ the parameter could not be detected in the clinical group.
Effect when treated with 10 mg methylphenidate.
Effect greater in participants with active tics and those not taking neuroleptic medication.
Corticospinal Tract
Complement to anatomic neuroimaging
Single-pulse TMS can detect corticospinal tract abnormalities not identified by anatomic neuroimaging. For example, single-pulse TMS was used to reveal subtle abnormalities in motor evoked potential duration variability in a boy with adrenoleukodystrophy and long-tract signs despite magnetic resonance imaging changes confined to the parieto-occipital white matter region.66 Similarly, single-pulse TMS has been used to detect neurophysiologic corticospinal tract abnormalities in children with multiple sclerosis and transverse myelitis who demonstrate no significant involvement of the motor system on magnetic resonance imaging.67,68 Indeed, single-pulse TMS may be useful to confirm the diagnosis of multiple sclerosis and estimate disease progression in adolescents and adults.69,70
Differentiating and diagnosing neurologic disorders
TMS may aid in narrowing the differential diagnosis of neurological symptoms by confirming the presence of corticospinal tract involvement in specific neurologic diseases. For example, single-pulse TMS may help in differentiating between ataxia syndromes that present in childhood. Corticospinal tract abnormalities were found in children with Friedreich’s ataxia and early onset recessive ataxia, but were less marked in early onset non-Friedreich’s ataxia and not present in autosomal dominant cerebellar ataxia.71–77 Single-pulse TMS has been useful in confirming and quantifying the presence of pyramidal signs in children with hereditary motor and sensory neuropathy types I and II78,79 and identifying the level of the lesion in infantile onset Pelizaeus-Merzbacher80 and familial childhood primary lateral sclerosis.81 Single-pulse TMS may also be useful in indicating the lack of corticospinal tract involvement in some pediatric neurological disorders, including childhood onset hereditary extrapyramidal disease,82 spinal muscular atrophy type II,83 and hereditary spastic paraplegia,78 thereby narrowing the differential diagnosis. Thus, the utility of single-pulse TMS in diagnosing and differentiating neurological disorders is promising in children. However, much of this evidence is based on a small number of patients in limited studies. Further studies should help demonstrate the utility of TMS in the differential diagnosis of specific pediatric neurologic disorders and establish sensitivity and specificity of this technique for differentiating specific neurologic disorders.
Indexing neurologic disease progression and treatment
TMS can aid in following the progression and resolution of neurologic disorders. Corticospinal tract changes appear to correlate with disease duration and progression in Friedreich’s ataxia72,73,75,76 and may be associated with the degree of metabolic control and microangiopathic complications in patients with longstanding insulin-dependent diabetes.84 Single-pulse TMS has been used to verify the efficacy of treatment. For example, single-pulse TMS was used to document improvement in corticospinal tract function after neck stabilization in Hirayama disease,85 D-penicillamine treatment in Wilson’s disease,86 and the lack of neurophysiological changes despite symptomatic improvement with digitalis treatment in infantile onset Pelizaeus-Merzbacher.87 Single-pulse TMS has been used to verify the efficacy of intrathecal baclofen in spasticity88 and the integrity of the corticospinal tract following scoliosis surgery.89 The resolution of corticospinal tract asymmetry in idiopathic congenital mirror movements90 and corticospinal abnormalities in transverse myelitis68 have also been documented with single-pulse TMS. Thus, TMS appears to be useful for monitoring the progression, resolution, and treatment of neurologic disease associated with corticospinal tract changes.
Insight into disease mechanism
Asymmetry in neurophysiological height-corrected motor evoked potential latency was detected using single-pulse TMS in untreated children with attention-deficit/hyperactivity disorder (ADHD), suggesting asymmetric hemispheric function, and single-pulse TMS detected prolonged central motor conduction time in children and adolescents with ADHD.91,92 Single-pulse TMS has been used to demonstrate that corticospinal tract abnormalities in childhood at the onset of alternating hemiplegia are reversible93 and that neuronal mechanisms of congenital mirror movements are neurophysiologically different than normal developmental mirror movements.94,95 Single-pulse TMS studies have demonstrated unique changes in neurophysiological corticospinal tract measurements in Rett syndrome that are consistent with the disorder’s neuropathology.96–99 Single-pulse TMS studies have demonstrated corticospinal tract abnormalities in malnourished children, suggesting possible delay in white matter myelination in such children.100,101
Cortical Excitability and Interhemispheric Dynamics
TMS studies suggest that measures of the cortical excitability can provide diagnostic information, aid in monitoring treatment efficiency, and provide insights into disease mechanism.
Epilepsy
TMS studies in children suggested that antiepileptic medications may elevate the motor threshold.102–105 At least one study demonstrated a higher motor threshold in partial epilepsy as compared to generalized epilepsy and control subjects.106 The motor threshold was found to be elevated in the affected hemisphere as compared to the unaffected hemisphere of a child with progressive focal epilepsy.107
Hemisphere excitability may depend on etiology since another study showed hyperexcitability in the affected hemisphere of a child with unilateral cortical dysplasia and intractable simple partial seizures, with this hyperexcitability reduced after multiple subpial transections.108 Cortical hyperexcitability has been suggested in benign epilepsy with centrotemporal spikes109 and progressive myoclonic epilepsy.110
Abnormal intracortical inhibition and/or facilitation have been consistently found in epilepsy. Intracortical inhibition was found to be reduced in progressive myoclonic epilepsy110,111 and the affected hemisphere in focal motor epilepsy.107 Intracortical facilitation was found to be increased in progressive myoclonic epilepsy111 and idiopathic generalized epilepsy.112 One study suggests that the amount of intracortical inhibition reduction and intracortical facilitation enhancement may be proportional to subclinical seizure activity.104
The silent period has been found to be prolonged in treated children with idiopathic generalized epilepsy113 and motor, but not nonmotor, cryptogenic partial epilepsy.114 This latter finding is asymmetric, showing greater prolongation in the limb contralateral to the normal hemisphere. Others have suggested that the silent period is reduced in the epileptic cortex.107 Prolongation of the silent period has been suggested to be related to seizure control115 and/or antiepileptic treatment.106
Thus, TMS has demonstrated that abnormalities in cortical excitability and interhemispheric dynamics are found in patients with epilepsy. Further studies are needed in order to determine how these parameters can be used to diagnose the type and severity of the epilepsy, guide the choice of the best antiepileptic agents, and monitor treatment efficacy.
Medical, neurologic, and psychiatric disease
Single-pulse TMS studies were the first to demonstrate cortical hyperexcitability in Rett syndrome.96 Duchenne muscular dystrophy and well-controlled phenylketonuria patients have been found to be associated with an elevated motor threshold despite normal corticospinal tract parameters.116,117 Intracortical inhibition was found to be reduced in ADHD118–120 and Tourette syndrome.121 Methylphenidate treatment was found to partially reverse intracortical inhibition abnormalities in children with ADHD,118,120 whereas such treatment has been found to enhance intracortical facilitation in normal adults.122 The cortical silent period was found to be reduced in controlled phenylketonuria patients,117 tic disorder,118,121,123 and ADHD.124
Using TMS to Detect Subtle Neurophysiological Abnormalities
The versatility of TMS allows exploration of static and dynamic aspects of cortical and corticospinal tract function in specific neurologic and medical disorders. TMS can detect corticospinal tract abnormalities that anatomic neuroimaging does not reveal, allowing the involvement of white matter tracts and the progression and resolution of such involvement to be followed. Alternatively, the confirmation or exclusion of corticospinal tract involvement in several neurologic syndromes may aid in differential diagnosis. TMS has been used to identify abnormalities in cortical excitability in epilepsy, myoclonus, tic disorder, attention deficit disorder, Tourette syndrome, and Rett syndrome. In addition to providing insights into the neurological mechanisms underlying these disorders, TMS may also aid in monitoring the efficacy of therapy.
Therapeutic Applications
Therapeutic applications of TMS have been growing in adult neurology. Below we briefly review some of the applications of TMS in adults and outline how such techniques could be applied to the pediatric population.
Stroke Recovery
TMS may have therapeutic utility for augmenting stroke recovery in children. In adults, TMS has been used to augment rehabilitation of motor function. TMS directed to the motor cortex combined with peripheral nerve stimulation, the so-called “dual stimulation” paradigm, results in changes in cortical excitability and motor output maps in normal participants that last hours and days, respectively.3,4 Several clinical parameters were found to improve in patients with chronic hemiplegia using such a paradigm.5 The enhanced neuroplasticity of the young brain may potentiate any therapeutic effect of TMS. Future studies will need to address “dual stimulation” therapy in children.
TMS may also be applicable for guiding neuroplasticity after stroke in children. Neuroimaging studies indicate that the best functional recovery from hemiplegia following stroke in adults is associated with the development of alternative pathways from the affected hemisphere to the affected limb without connections from the unaffected hemisphere to the affected limb.64,125 Authors have suggested that the ipsilateral hemispheric projections during the acute and subacute poststroke period represent a transient stage of reorganization in normal recovery. However, optimal functional recovery appears to involve attenuation of temporary ipsilateral hemisphere function with concomitant reorganization of the affected hemisphere. Chronic continuation of ipsilateral hemisphere motor function could induce interhemispheric rivalry, thereby impeding optimal functional recovery.
TMS has been used to experimentally demonstrate hemispheric rivalry in normal subjects.126 TMS has been used to treat nonmotor neurologic deficits that presumably result from hemispheric rivalry in adults with acquired unilateral brain lesions. For example, single-pulse TMS over the left frontal or parietal cortices improved tactile extinction in patients with right cortical damage.127,128 Naeser and Pascual-Leone applied low-frequency repetitive TMS to the right supplementary motor area of chronic Broca’s aphasia patients based on functional neuroimaging data showing overactivity in this brain region.129 This TMS treatment appeared to significantly improve confrontational naming with this effect sustained up to 8 months following the treatment.130,131 Presumably, TMS released the residual function of a unilateral damaged cortical area by dampening excessive interhemispheric inhibition.
It is not known how patterns of reorganization in the adult correlate with cortical reorganization in the child, but the evidence from adult studies provides guidance for studying and understanding brain reorganization after brain injury in children. Evidence suggests that TMS may be beneficial in guiding neuroplasticity in adults. Since neuroplasticity is believed to be more prominent in the pediatric brain, such therapies may be more successful in children.
Psychiatric Disorders
TMS has been applied to bipolar and chronic depression, anxiety, obsessive-compulsive disorder, and schizophrenia in adults using various protocols with a variety of successes.6 Although the TMS protocol and patient selection need to be more clearly defined, the data from many adult studies are promising. Data from the pediatric population are limited. Five of seven older adolescents with bipolar depression, unipolar depression, or schizophrenia responded to therapeutic TMS protocols.132 The ability of TMS to guide neuroplasticity is exciting in the area of psychiatry since TMS therapy could be used to modify abnormally functioning circuits before chronic changes are established. Thus, preliminary applications of TMS in children and adolescents are encouraging, but larger case series and, eventually, controlled trials are needed.
Epilepsy
Early studies attempted to use TMS to induce seizures in epilepsy patients. These studies documented the poor efficacy of TMS as a tool for inducing such seizures.133 This may have been due to a therapeutic effect of TMS on epilepsy. Indeed, low-frequency repetitive TMS may have a therapeutic effect on epileptic discharges and seizures. This has been demonstrated in animal models134 and mesiotemporal epilepsy.135 Uncontrolled human studies have reported improvement in progressive myoclonic epilepsy7 and intractable focal epilepsy8,9 following low-frequency repetitive TMS. High-frequency repetitive TMS may also have utility in modulating the activity of seizure foci. For example, focal hyperperfusion, as measured by single photon emission computed tomography, was reversed in 2 patients with epilepsia partialis continua with one application of high-frequency repetitive TMS.136
Several controlled studies have examined the efficacy of low-frequency repetitive TMS on seizure control in adults. A controlled study demonstrated a nonsignificant decrease in seizure frequency in intractable focal epilepsy after 1 week of daily low-frequency repetitive TMS. Patients with neocortical foci had a greater response than patients with mesial temporal foci, suggesting that focal cortical abnormalities may be more responsive to TMS treatment.137 Indeed, 2 of the most successful studies appear to suggest that targeting a cortical focus is efficacious. In an open study, Fregni et al.138 targeted cortical malformations in 8 patients with refractory epilepsy in one session of low-frequency repetitive TMS. Compared to a 4-week baseline preceding the treatment, this treatment significantly reduced the number of epileptiform discharges as measured by electroencephalogram and the number of self-reported seizures. In a follow-up study Fregni et al.139 applied 5 sessions of low-frequency repetitive TMS in a randomized double-blind sham-controlled fashion. The number of reported seizures in the treatment group was significantly lower up to 2 months following the treatment. Certain aspects of cognition also improved in the treatment group. However, Joo et al.140 found that 2 different doses of low-frequency repetitive TMS applied to focal or multifocal epilepsy patients reduced the number of epileptiform discharges, but did not reduce the number of clinical seizures. These researchers did not find any difference in the reduction in the number of seizures or epileptiform discharges between the focal and multifocal groups.
Although the utility for TMS in seizure control is encouraging, further evidence of efficacy will be needed before this technology can be applied clinically. Differences in application protocols, seizure etiology, and the differential therapeutic effect of TMS on different seizure types require specific attention. These studies verify the safety of TMS in epileptic patients. A mature therapy for focal epilepsies will indeed be highly beneficial in children. Thus, pediatric trials will hopefully begin soon.
Learning Disabilities
As mentioned above, TMS has been used to treat neurologic deficits that presumably result from hemispheric rivalry in adults with acquired unilateral brain lesions. Like the chronic Broca’s aphasia patients described above, children with dyslexia and young children at risk for reading disorders show reverse lateralization of cortical activity, particularly in the temporoparietal area, during language processing.141 Although language training can ameliorate some of the abnormal cortical activity in the right temporoparietal area, lateralization of cortical activity usually remains abnormal.142 Application of low-frequency repetitive TMS to overactive cortical regions in dyslexic individuals could promote normal lateralization and release any inhibitory influence on the left underactive language areas from hemispheric rivalry mechanisms. Alternatively, since high-frequency repetitive TMS and single-pulse TMS can enhance cognitive performance,143–146 TMS protocols could be developed to enhance cognitive function for children with specific learning disabilities by exciting underactive neural pathways.
TMS Safety in Children
TMS has been reportedly used in over 800 normal children and over 300 neurologically abnormal children, including at least 25 children with epilepsy. Several authors have highlighted the absence of reported adverse effects in children.10,11 The 84 TMS studies in which the cortex of children was stimulated with TMS were reviewed: 15 mentioned that no side effects occurred, one mentioned that one patient had an adverse reaction to the protocol,132 and another study mentioned that the patient experienced transient dullness in the left upper limb.85 The remainder of the studies did not comment on side effects.
The maximum charge density of TMS is markedly below the level at which neural damage can occur,147 and no microscopic brain damage could be found after hundreds of TMS stimuli to rat, rabbit, or human brains.148–150 In adults, TMS safety studies have examined the electroencephalogram and cognitive, audiologic, and hormonal parameters,151–153 and safety guidelines have been developed.154 In children, no change in auditory function as assessed by brain stem auditory evoked potentials, otoacoustic emissions, acoustic reflex, and a pure tone audiometric and logoaudiometric was found after TMS without ear protection.155 Safety guidelines need to be developed for TMS use in children.
Summary
From the review of the current uses of TMS in children, we can see the great potential for TMS in the future for the pediatric population. TMS can (1) provide an index of maturation of the cortex and corticospinal pathway, (2) detect patterns of CNS reorganization following brain damage, (3) help diagnosis and follow neurological and medical diseases, (4) provide insight into disease mechanisms, and (5) monitor the efficacy of treatment. In addition, TMS is being developed as a nonpharmacologic therapeutic intervention for several neurologic and psychiatric conditions in adults, such as stroke,3–5 major depression,6 and epilepsy.7–9 TMS has been shown to directly modulate neuroplasticity in adults with effects that last minutes to days to months. Since the pediatric brain is thought to be significantly more plastic than the adult brain, TMS therapy could result in neuroplasticity changes that may be longer lasting. Clearly, the potential utility of this technique in pediatric neurology is significant, although attention to safety is critical. Hopefully, more studies will be designed to apply this technique to the pediatric population, especially with its excellent safety profile in this population.
Acknowledgments
The authors would like to thank Ian Butler, MD, and Tony Ro, PhD, for their insightful comments on earlier versions of this manuscript.
This research was supported by K23 NS046565 from NINDS to Dr. Richard Frye and K24 RR018875 to Alvaro Pascual-Leone. None of the authors declare a conflict of interest or commercial support.
References
- 1.Kobayashi M, Pascual-Leone A. Transcranial magnetic stimulation in neurology. Lancet Neurol. 2003;2:145–156. doi: 10.1016/s1474-4422(03)00321-1. [DOI] [PubMed] [Google Scholar]
- 2.Pascual-Leone A, Walsh V. Transcranial magnetic simulation. In: Toga A, Mazziotta J, editors. Brain Mapping: The Methods. San Diego, CA: Academic Press; 2002. pp. 255–290. [Google Scholar]
- 3.Stefan K, Kunesch E, Cohen LG, et al. Induction of plasticity in the human motor cortex by paired associative stimulation. Brain. 2000;123:572–584. doi: 10.1093/brain/123.3.572. [DOI] [PubMed] [Google Scholar]
- 4.McKay DR, Ridding MC, Thompson PD, et al. Induction of persistent changes in the organization of the human motor cortex. Exp Brain Res. 2002;143:342–349. doi: 10.1007/s00221-001-0995-3. [DOI] [PubMed] [Google Scholar]
- 5.Uy J, Ridding MC. Increased cortical excitability induced by transcranial DC and peripheral nerve stimulation. J Neurosci Methods. 2003;127:193–197. doi: 10.1016/s0165-0270(03)00142-0. [DOI] [PubMed] [Google Scholar]
- 6.Lisanby SH, Kinnunen LH, Crupain MJ. Applications of TMS to therapy in psychiatry. J Clin Neurophysiol. 2002;19:344–360. doi: 10.1097/00004691-200208000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Wedegaertner FR, Garvey MA, Cohen LG, et al. Low frequency repetitive transcranial magnetic stimulation can reduce action myoclonus. Neurology. 1997;48:A119. [Google Scholar]
- 8.Menkes DL, Gruenthal M. Slow-frequency repetitive transcranial magnetic stimulation in a patient with focal cortical dysplasia. Epilepsia. 2000;41:240–242. doi: 10.1111/j.1528-1157.2000.tb00146.x. [DOI] [PubMed] [Google Scholar]
- 9.Tergau F, Naumann U, Paulus W, et al. Low-frequency repetitive transcranial magnetic stimulation improves intractable epilepsy. Lancet. 1999;353:2209. doi: 10.1016/S0140-6736(99)01301-X. [DOI] [PubMed] [Google Scholar]
- 10.Quintana H. Transcranial magnetic stimulation in persons younger than the age of 18. J ECT. 2005;21:88–95. doi: 10.1097/01.yct.0000162556.02720.58. [DOI] [PubMed] [Google Scholar]
- 11.Garvey MA, Gilbert DL. Transcranial magnetic stimulation in children. Eur J Paediatr Neurol. 2004;8:7–19. doi: 10.1016/j.ejpn.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 12.Gilbert DL, Garvey MA, Bansal AS, et al. Should transcranial magnetic stimulation research in children be considered minimal risk? Clin Neurophysiol. 2004;115:1730–1739. doi: 10.1016/j.clinph.2003.10.037. [DOI] [PubMed] [Google Scholar]
- 13.Barker AT. An introduction to the basic principles of magnetic nerve stimulation. J Clin Neurophysiol. 1991;8:26–37. doi: 10.1097/00004691-199101000-00005. [DOI] [PubMed] [Google Scholar]
- 14.Jalinous R. Technical and practical aspects of magnetic nerve stimulation. J Clin Neurophysiol. 1991;8:10–25. doi: 10.1097/00004691-199101000-00004. [DOI] [PubMed] [Google Scholar]
- 15.Pascual-Leone A, Bartres-Faz D, Keenan JP. Transcranial magnetic stimulation: studying the brain-behaviour relationship by induction of ‘virtual lesions’. Philos Trans R Soc Lond B Biol Sci. 1999;354:1229–1238. doi: 10.1098/rstb.1999.0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth BJ, Saypol JM, Hallett M, et al. A theoretical calculation of the electric field induced in the cortex during magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1991;81:47–56. doi: 10.1016/0168-5597(91)90103-5. [DOI] [PubMed] [Google Scholar]
- 17.Liepert J, Dettmers C, Terborg C, et al. Inhibition of ipsilateral motor cortex during phasic generation of low force. Clin Neurophysiol. 2001;112:114–121. doi: 10.1016/s1388-2457(00)00503-4. [DOI] [PubMed] [Google Scholar]
- 18.Largo RH, Caflisch JA, Hug F, et al. Neuromotor development from 5 to 18 years. Part 2: associated movements. Dev Med Child Neurol. 2001;43:444–453. doi: 10.1017/s0012162201000822. [DOI] [PubMed] [Google Scholar]
- 19.Largo RH, Caflisch JA, Hug F, et al. Neuromotor development from 5 to 18 years. Part 1: timed performance. Dev Med Child Neurol. 2001;43:436–443. doi: 10.1017/s0012162201000810. [DOI] [PubMed] [Google Scholar]
- 20.Webster RI, Erdos C, Evans K, et al. The clinical spectrum of developmental language impairment in school-aged children: language, cognitive, and motor findings. Pediatrics. 2006;118:e1541–1549. doi: 10.1542/peds.2005-2761. [DOI] [PubMed] [Google Scholar]
- 21.Kutschera J, Tomaselli J, Maurer U, et al. Minor neurological dysfunction, cognitive development and somatic development at the age of 3 to 11 years in very-low-birthweight infants with transient periventricular echodensities. Acta Paediatr. 2006;95:1577–1581. doi: 10.1080/08035250600643236. [DOI] [PubMed] [Google Scholar]
- 22.Burns Y, O’Callaghan M, McDonell B, et al. Movement and motor development in ELBW infants at 1 year is related to cognitive and motor abilities at 4 years. Early Hum Dev. 2004;80:19–29. doi: 10.1016/j.earlhumdev.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Jeyaseelan D, O’Callaghan M, Neulinger K, et al. The association between early minor motor difficulties in extreme low birth weight infants and school age attentional difficulties. Early Hum Dev. 2006;82:249–255. doi: 10.1016/j.earlhumdev.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 24.Murray GK, Veijola J, Moilanen K, et al. Infant motor development is associated with adult cognitive categorization in a longitudinal birth cohort study. J Child Psychol Psychiatry. 2006;47:25–29. doi: 10.1111/j.1469-7610.2005.01450.x. [DOI] [PubMed] [Google Scholar]
- 25.Eyre JA, Taylor JP, Villagra F, et al. Evidence of activity-dependent withdrawal of corticospinal projections during human development. Neurology. 2001;57:1543–1554. doi: 10.1212/wnl.57.9.1543. [DOI] [PubMed] [Google Scholar]
- 26.Masur H, Althoff S, Kurlemann G, et al. Inhibitory period and late muscular responses after transcranial magnetic stimulation in healthy children. Brain Dev. 1995;17:149–152. doi: 10.1016/0387-7604(94)00124-g. [DOI] [PubMed] [Google Scholar]
- 27.Muller K, Homberg V, Lenard HG. Magnetic stimulation of motor cortex and nerve roots in children. Maturation of cortico-motoneuronal projections. Electroencephalogr Clin Neurophysiol. 1991;81:63–70. doi: 10.1016/0168-5597(91)90105-7. [DOI] [PubMed] [Google Scholar]
- 28.Nezu A, Kimura S, Uehara S, et al. Magnetic stimulation of motor cortex in children: maturity of corticospinal pathway and problem of clinical application. Brain Dev. 1997;19:176–180. doi: 10.1016/s0387-7604(96)00552-9. [DOI] [PubMed] [Google Scholar]
- 29.Heinen F, Glocker FX, Fietzek U, et al. Absence of transcallosal inhibition following focal magnetic stimulation in preschool children. Ann Neurol. 1998;43:608–612. doi: 10.1002/ana.410430508. [DOI] [PubMed] [Google Scholar]
- 30.Mall V, Berweck S, Fietzek UM, et al. Low level of intracortical inhibition in children shown by transcranial magnetic stimulation. Neuropediatrics. 2004;35:120–125. doi: 10.1055/s-2004-815834. [DOI] [PubMed] [Google Scholar]
- 31.Moll GH, Heinrich H, Wischer S, et al. Motor system excitability in healthy children: developmental aspects from transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol Suppl. 1999;51:243–249. [PubMed] [Google Scholar]
- 32.Fietzek UM, Heinen F, Berweck S, et al. Development of the corticospinal system and hand motor function: central conduction times and motor performance tests. Dev Med Child Neurol. 2000;42:220–227. doi: 10.1017/s0012162200000384. [DOI] [PubMed] [Google Scholar]
- 33.Heinen F, Fietzek UM, Berweck S, et al. Fast corticospinal system and motor performance in children: conduction proceeds skill. Pediatr Neurol. 1998;19:217–221. doi: 10.1016/s0887-8994(98)00057-5. [DOI] [PubMed] [Google Scholar]
- 34.Nezu A, Kimura S, Takeshita S. Topographical differences in the developmental profile of central motor conduction time. Clin Neurophysiol. 1999;110:1646–1649. doi: 10.1016/s1388-2457(99)00118-2. [DOI] [PubMed] [Google Scholar]
- 35.Muller K, Homberg V. Development of speed of repetitive movements in children is determined by structural changes in corticospinal efferents. Neurosci Lett. 1992;144:57–60. doi: 10.1016/0304-3940(92)90715-j. [DOI] [PubMed] [Google Scholar]
- 36.Muller K, Kass-Iliyya F, Reitz M. Ontogeny of ipsilateral corticospinal projections: a developmental study with transcranial magnetic stimulation. Ann Neurol. 1997;42:705–711. doi: 10.1002/ana.410420506. [DOI] [PubMed] [Google Scholar]
- 37.Caramia MD, Desiato MT, Cicinelli P, et al. Latency jump of “relaxed” versus “contracted” motor evoked potentials as a marker of cortico-spinal maturation. Electroencephalogr Clin Neurophysiol. 1993;89:61–66. doi: 10.1016/0168-5597(93)90086-5. [DOI] [PubMed] [Google Scholar]
- 38.Koh TH, Eyre JA. Maturation of corticospinal tracts assessed by electromagnetic stimulation of the motor cortex. Arch Dis Child. 1988;63:1347–1352. doi: 10.1136/adc.63.11.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Garvey MA, Ziemann U, Bartko JJ, et al. Cortical correlates of neuromotor development in healthy children. Clin Neurophysiol. 2003;114:1662–1670. doi: 10.1016/s1388-2457(03)00130-5. [DOI] [PubMed] [Google Scholar]
- 40.Heinen F, Kirschner J, Fietzek U, et al. Absence of transcallosal inhibition in adolescents with diplegic cerebral palsy. Muscle Nerve. 1999;22:255–257. doi: 10.1002/(sici)1097-4598(199902)22:2<255::aid-mus14>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 41.Bender S, Basseler K, Sebastian I, et al. Electroencephalographic response to transcranial magnetic stimulation in children: evidence for giant inhibitory potentials. Ann Neurol. 2005;58:58–67. doi: 10.1002/ana.20521. [DOI] [PubMed] [Google Scholar]
- 42.Kozel FA, Nahas Z, deBrux C, et al. How coil-cortex distance relates to age, motor threshold, and antidepressant response to repetitive transcranial magnetic stimulation. J Neuropsychiatry Clin Neurosci. 2000;12:376–384. doi: 10.1176/jnp.12.3.376. [DOI] [PubMed] [Google Scholar]
- 43.McConnell KA, Nahas Z, Shastri A, et al. The transcranial magnetic stimulation motor threshold depends on the distance from coil to underlying cortex: a replication in healthy adults comparing two methods of assessing the distance to cortex. Biol Psychiatry. 2001;49:454–459. doi: 10.1016/s0006-3223(00)01039-8. [DOI] [PubMed] [Google Scholar]
- 44.Eyre JA, Miller S, Ramesh V. Constancy of central conduction delays during development in man: investigation of motor and somatosensory pathways. J Physiol. 1991;434:441–452. doi: 10.1113/jphysiol.1991.sp018479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Eyre JA, Miller S, Clowry GJ. The development of the corticospinal tract in humans. In: Pascual-Leone A, Davey NJ, Rothwell J, et al., editors. Handbook of Transcranial Magnetic Stimulation. New York, NY: Oxford University Press; 2002. pp. 235–249. [Google Scholar]
- 46.Chiappa KH, Cros D, Day BL, et al. Magnetic simulation of the human motor cortex: ipsilateral and contralateral facilitation effects. In: Levy WJ, Cracco RQ, Barker AT, et al., editors. Magnetic Motor Stimulation: Basic Principles and Clinical Experience. Amsterdam: Elsevier; 1991. pp. 186–201. [PubMed] [Google Scholar]
- 47.Wagner T, Gangitano M, Romero R, et al. Intracranial measurement of current densities induced by transcranial magnetic stimulation in the human brain. Neurosci Lett. 2004;354:91–94. doi: 10.1016/s0304-3940(03)00861-9. [DOI] [PubMed] [Google Scholar]
- 48.Wagner T, Valero-Cabre A, Pascual-Leone A. Noninvasive human brain stimulation. Annu Rev Biomed Eng. 2007;9:527–565. doi: 10.1146/annurev.bioeng.9.061206.133100. [DOI] [PubMed] [Google Scholar]
- 49.Nezu A, Kimura S, Takeshita S, et al. Functional recovery in hemiplegic cerebral palsy: ipsilateral electromyographic responses to focal transcranial magnetic stimulation. Brain Dev. 1999;21:162–165. doi: 10.1016/s0387-7604(98)00094-1. [DOI] [PubMed] [Google Scholar]
- 50.Carr LJ. Development and reorganization of descending motor pathways in children with hemiplegic cerebral palsy. Acta Paediatr Suppl. 1996;416:53–57. doi: 10.1111/j.1651-2227.1996.tb14278.x. [DOI] [PubMed] [Google Scholar]
- 51.Carr LJ, Harrison LM, Evans AL, et al. Patterns of central motor reorganization in hemiplegic cerebral palsy. Brain. 1993;116:1223–1247. doi: 10.1093/brain/116.5.1223. [DOI] [PubMed] [Google Scholar]
- 52.Staudt M, Grodd W, Gerloff C, et al. Two types of ipsilateral reorganization in congenital hemiparesis: a TMS and fMRI study. Brain. 2002;125:2222–2237. doi: 10.1093/brain/awf227. [DOI] [PubMed] [Google Scholar]
- 53.Farmer SF, Harrison LM, Ingram DA, et al. Plasticity of central motor pathways in children with hemiplegic cerebral palsy. Neurology. 1991;41:1505–1510. doi: 10.1212/wnl.41.9.1505. [DOI] [PubMed] [Google Scholar]
- 54.Maegaki Y, Yamamoto T, Takeshita K. Plasticity of central motor and sensory pathways in a case of unilateral extensive cortical dysplasia: investigation of magnetic resonance imaging, transcranial magnetic stimulation, and short-latency somatosensory evoked potentials. Neurology. 1995;45:2255–2261. doi: 10.1212/wnl.45.12.2255. [DOI] [PubMed] [Google Scholar]
- 55.Maegaki Y, Maeoka Y, Ishii S, et al. Mechanisms of central motor reorganization in pediatric hemiplegic patients. Neuropediatrics. 1997;28:168–174. doi: 10.1055/s-2007-973695. [DOI] [PubMed] [Google Scholar]
- 56.Thickbroom GW, Byrnes ML, Archer SA, et al. Differences in sensory and motor cortical organization following brain injury early in life. Ann Neurol. 2001;49:320–327. [PubMed] [Google Scholar]
- 57.Maegaki Y, Maeoka Y, Ishii S, et al. Central motor reorganization in cerebral palsy patients with bilateral cerebral lesions. Pediatr Res. 1999;45:559–567. doi: 10.1203/00006450-199904010-00016. [DOI] [PubMed] [Google Scholar]
- 58.Hamzei F, Liepert J, Dettmers C, et al. Structural and functional cortical abnormalities after upper limb amputation during childhood. Neuroreport. 2001;12:957–962. doi: 10.1097/00001756-200104170-00019. [DOI] [PubMed] [Google Scholar]
- 59.Roricht S, Machetanz J, Irlbacher K, et al. Reorganization of human motor cortex after hand replantation. Ann Neurol. 2001;50:240–249. doi: 10.1002/ana.1091. [DOI] [PubMed] [Google Scholar]
- 60.Alagona G, Delvaux V, Gerard P, et al. Ipsilateral motor responses to focal transcranial magnetic stimulation in healthy subjects and acute-stroke patients. Stroke. 2001;32:1304–1309. doi: 10.1161/01.str.32.6.1304. [DOI] [PubMed] [Google Scholar]
- 61.Cruz Martinez A, Tejada J, Diez Tejedor E. Motor hand recovery after stroke. Prognostic yield of early transcranial magnetic stimulation. Electromyogr Clin Neurophysiol. 1999;39:405–410. [PubMed] [Google Scholar]
- 62.Escudero JV, Sancho J, Bautista D, et al. Prognostic value of motor evoked potential obtained by transcranial magnetic brain stimulation in motor function recovery in patients with acute ischemic stroke. Stroke. 1998;29:1854–1859. doi: 10.1161/01.str.29.9.1854. [DOI] [PubMed] [Google Scholar]
- 63.Pennisi G, Rapisarda G, Bella R, et al. Absence of response to early transcranial magnetic stimulation in ischemic stroke patients: prognostic value for hand motor recovery. Stroke. 1999;30:2666–2670. doi: 10.1161/01.str.30.12.2666. [DOI] [PubMed] [Google Scholar]
- 64.Trompetto C, Assini A, Buccolieri A, et al. Motor recovery following stroke: a transcranial magnetic stimulation study. Clin Neurophysiol. 2000;111:1860–1867. doi: 10.1016/s1388-2457(00)00419-3. [DOI] [PubMed] [Google Scholar]
- 65.Rossini PM. Is transcranial magnetic stimulation of the motor cortex a prognostic tool for motor recovery after stroke? Stroke. 2000;31:1463–1464. doi: 10.1161/01.str.31.6.1457-d. [DOI] [PubMed] [Google Scholar]
- 66.Nezu A, Kimura S, Kobayashi T, et al. Transcranial magnetic stimulation in an adrenoleukodystrophy patient. Brain Dev. 1996;18:327–329. doi: 10.1016/0387-7604(96)00011-3. [DOI] [PubMed] [Google Scholar]
- 67.Dan B, Christiaens F, Christophe C, et al. Transcranial magnetic stimulation and other evoked potentials in pediatric multiple sclerosis. Pediatr Neurol. 2000;22:136–138. doi: 10.1016/s0887-8994(99)00111-3. [DOI] [PubMed] [Google Scholar]
- 68.Noguchi Y, Okubo O, Fuchigami T, et al. Motor-evoked potentials in a child recovering from transverse myelitis. Pediatr Neurol. 2000;23:436–438. doi: 10.1016/s0887-8994(00)00211-3. [DOI] [PubMed] [Google Scholar]
- 69.Cruz-Martinez A, Gonzalez-Orodea JI, Lopez Pajares R, et al. Disability in multiple sclerosis. The role of transcranial magnetic stimulation. Electromyogr Clin Neurophysiol. 2000;40:441–447. [PubMed] [Google Scholar]
- 70.Schmierer K, Irlbacher K, Grosse P, et al. Correlates of disability in multiple sclerosis detected by transcranial magnetic stimulation. Neurology. 2002;59:1218–1224. doi: 10.1212/wnl.59.8.1218. [DOI] [PubMed] [Google Scholar]
- 71.Claus D, Harding AE, Hess CW, et al. Central motor conduction in degenerative ataxic disorders: a magnetic stimulation study. J Neurol Neurosurg Psychiatry. 1988;51:790–795. doi: 10.1136/jnnp.51.6.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cruz Martinez A, Anciones B. Central motor conduction to upper and lower limbs after magnetic stimulation of the brain and peripheral nerve abnormalities in 20 patients with Friedreich’s ataxia. Acta Neurol Scand. 1992;85:323–326. doi: 10.1111/j.1600-0404.1992.tb04051.x. [DOI] [PubMed] [Google Scholar]
- 73.Cruz-Martinez A, Palau F. Central motor conduction time by magnetic stimulation of the cortex and peripheral nerve conduction follow-up studies in Friedreich’s ataxia. Electroencephalogr Clin Neurophysiol. 1997;105:458–461. doi: 10.1016/s0924-980x(97)00047-7. [DOI] [PubMed] [Google Scholar]
- 74.Lanzillo B, Perretti A, Santoro L, et al. Evoked potentials in inherited ataxias: a multimodal electrophysiological study. Ital J Neurol Sci. 1994;15:25–37. doi: 10.1007/BF02343494. [DOI] [PubMed] [Google Scholar]
- 75.Mondelli M, Rossi A, Scarpini C, et al. Motor evoked potentials by magnetic stimulation in hereditary and sporadic ataxia. Electromyogr Clin Neurophysiol. 1995;35:415–424. [PubMed] [Google Scholar]
- 76.Santoro L, Perretti A, Lanzillo B, et al. Influence of GAA expansion size and disease duration on central nervous system impairment in Friedreich’s ataxia: contribution to the understanding of the pathophysiology of the disease. Clin Neurophysiol. 2000;111:1023–1030. doi: 10.1016/s1388-2457(00)00290-x. [DOI] [PubMed] [Google Scholar]
- 77.Schwenkreis P, Tegenthoff M, Witscher K, et al. Motor cortex activation by transcranial magnetic stimulation in ataxia patients depends on the genetic defect. Brain. 2002;125:301–309. doi: 10.1093/brain/awf023. [DOI] [PubMed] [Google Scholar]
- 78.Claus D, Waddy HM, Harding AE, et al. Hereditary motor and sensory neuropathies and hereditary spastic paraplegia: a magnetic stimulation study. Ann Neurol. 1990;28:43–49. doi: 10.1002/ana.410280109. [DOI] [PubMed] [Google Scholar]
- 79.Sartucci F, Piaggesi A, Logi F, et al. Impaired ascendant central pathways conduction in impotent diabetic subjects. Acta Neurol Scand. 1999;99:381–386. doi: 10.1111/j.1600-0404.1999.tb07368.x. [DOI] [PubMed] [Google Scholar]
- 80.Nezu A, Kimura S, Takeshita S, et al. Magnetic stimulation of the corticospinal tracts in Pelizaeus-Merzbacher disease. Electroencephalogr Clin Neurophysiol. 1998;108:446–448. doi: 10.1016/s0168-5597(98)00018-5. [DOI] [PubMed] [Google Scholar]
- 81.Gascon GG, Chavis P, Yaghmour A, et al. Familial childhood primary lateral sclerosis with associated gaze paresis. Neuropediatrics. 1995;26:313–319. doi: 10.1055/s-2007-979781. [DOI] [PubMed] [Google Scholar]
- 82.Muller K, Homberg V, Aulich A, et al. Magnetoelectrical stimulation of motor cortex in children with motor disturbances. Electroencephalogr Clin Neurophysiol. 1992;85:86–94. doi: 10.1016/0168-5597(92)90073-k. [DOI] [PubMed] [Google Scholar]
- 83.Imai T, Matsuya M, Matsumoto H, et al. Preservation of central motor conduction in patients with spinal muscular atrophy type II. Brain Dev. 1995;17:432–435. doi: 10.1016/0387-7604(95)00087-9. [DOI] [PubMed] [Google Scholar]
- 84.d’Annunzio G, Moglia A, Zandrini C, et al. Central motor conduction time in children and adolescents with insulin-dependent diabetes mellitus (IDDM) Diabetes Res Clin Pract. 1995;28:57–62. doi: 10.1016/0168-8227(95)01062-i. [DOI] [PubMed] [Google Scholar]
- 85.Shizukawa H, Imai T, Kobayashi N, et al. Cervical flexion-induced changes of motor evoked potentials by transcranial magnetic stimulation in a patient with Hirayama disease: juvenile muscular atrophy of unilateral upper extremity. Rinsho Shinkeigaku. 1994;34:500–503. [PubMed] [Google Scholar]
- 86.Meyer BU, Britton TC, Benecke R. Wilson’s disease: normalisation of cortically evoked motor responses with treatment. J Neurol. 1991;238:327–30. doi: 10.1007/BF00315332. [DOI] [PubMed] [Google Scholar]
- 87.Nezu A, Kimura S, Osaka H, et al. Effect of digitalis on conduction dysfunction in Pelizaeus-Merzbacher disease. J Neurol Sci. 1996;141:49–53. doi: 10.1016/0022-510x(96)00134-7. [DOI] [PubMed] [Google Scholar]
- 88.Dachy B, Dan B. Electrophysiological assessment of the effect of intrathecal baclofen in spastic children. Clin Neurophysiol. 2002;113:336–340. doi: 10.1016/s1388-2457(02)00010-x. [DOI] [PubMed] [Google Scholar]
- 89.Tabaraud F, Boulesteix JM, Moulies D, et al. Monitoring of the motor pathway during spinal surgery. Spine. 1993;18:546–550. doi: 10.1097/00007632-199304000-00005. [DOI] [PubMed] [Google Scholar]
- 90.Maegaki Y, Seki A, Suzaki I, et al. Congenital mirror movement: a study of functional MRI and transcranial magnetic stimulation. Dev Med Child Neurol. 2002;44:838–843. doi: 10.1017/s0012162201003012. [DOI] [PubMed] [Google Scholar]
- 91.Ucles P, Lorente S, Rosa F. Neurophysiological methods testing the psychoneural basis of attention deficit hyperactivity disorder. Childs Nerv Syst. 1996;12:215–217. doi: 10.1007/BF00301253. [DOI] [PubMed] [Google Scholar]
- 92.Ucles P, Serrano JL, Rosa F. Central conduction time of magnetic brain stimulation in attention-deficit hyperactivity disorder. J Child Neurol. 2000;15:723–728. doi: 10.1177/088307380001501103. [DOI] [PubMed] [Google Scholar]
- 93.Nezu A, Kimura S, Ohtsuki N, et al. Alternating hemiplegia of childhood: report of a case having a long history. Brain Dev. 1997;19:217–221. doi: 10.1016/s0387-7604(96)00565-7. [DOI] [PubMed] [Google Scholar]
- 94.Reitz M, Muller K. Differences between ‘congenital mirror movements’ and ‘associated movements’ in normal children: a neurophysiological case study. Neurosci Lett. 1998;256:69–72. doi: 10.1016/s0304-3940(98)00748-4. [DOI] [PubMed] [Google Scholar]
- 95.Cincotta M, Lori S, Gangemi PF, et al. Hand motor cortex activation in a patient with congenital mirror movements: a study of the silent period following focal transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1996;101:240–246. doi: 10.1016/0924-980x(96)95621-0. [DOI] [PubMed] [Google Scholar]
- 96.Eyre JA, Kerr AM, Miller S, et al. Neurophysiological observations on corticospinal projections to the upper limb in subjects with Rett syndrome. J Neurol Neurosurg Psychiatry. 1990;53:874–879. doi: 10.1136/jnnp.53.10.874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Heinen F, Korinthenberg R. Does transcranial magnetic stimulation allow early diagnosis of Rett syndrome? Neuropediatrics. 1996;27:223–224. doi: 10.1055/s-2007-973794. [DOI] [PubMed] [Google Scholar]
- 98.Heinen F, Petersen H, Fietzek U, et al. Transcranial magnetic stimulation in patients with Rett syndrome: preliminary results. Eur Child Adolesc Psychiatry. 1997;6(suppl 1):S61–S63. [PubMed] [Google Scholar]
- 99.Nezu A, Kimura S, Takeshita S, et al. Characteristic response to transcranial magnetic stimulation in Rett syndrome. Electroencephalogr Clin Neurophysiol. 1998;109:100–103. doi: 10.1016/s0924-980x(97)00081-7. [DOI] [PubMed] [Google Scholar]
- 100.Karak B, Misra S, Garg RK, et al. A study of transcranial magnetic stimulation in older (>3 years) patients of malnutrition. Neurol India. 1999;47:229–233. [PubMed] [Google Scholar]
- 101.Tamer SK, Misra S, Jaiswal S. Central motor conduction time in malnourished children. Arch Dis Child. 1997;77:323–325. doi: 10.1136/adc.77.4.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Nezu A, Kimura S, Ohtsuki N, et al. Transcranial magnetic stimulation in benign childhood epilepsy with centro-temporal spikes. Brain Dev. 1997;19:134–137. doi: 10.1016/s0387-7604(96)00497-4. [DOI] [PubMed] [Google Scholar]
- 103.Michelucci R, Passarelli D, Riguzzi P, et al. Transcranial magnetic stimulation in partial epilepsy: drug-induced changes of motor excitability. Acta Neurol Scand. 1996;94:24–30. doi: 10.1111/j.1600-0404.1996.tb00034.x. [DOI] [PubMed] [Google Scholar]
- 104.Cantello R, Civardi C, Cavalli A, et al. Cortical excitability in cryptogenic localization-related epilepsy: interictal transcranial magnetic stimulation studies. Epilepsia. 2000;41:694–704. doi: 10.1111/j.1528-1157.2000.tb00230.x. [DOI] [PubMed] [Google Scholar]
- 105.Reutens DC, Puce A, Berkovic SF. Cortical hyperexcitability in progressive myoclonus epilepsy: a study with transcranial magnetic stimulation. Neurology. 1993;43:186–192. doi: 10.1212/wnl.43.1_part_1.186. [DOI] [PubMed] [Google Scholar]
- 106.Tataroglu C, Ozkiziltan S, Baklan B. Motor cortical thresholds and cortical silent periods in epilepsy. Seizure. 2004;13:481–485. doi: 10.1016/j.seizure.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 107.Inghilleri M, Mattia D, Berardelli A, et al. Asymmetry of cortical excitability revealed by transcranial stimulation in a patient with focal motor epilepsy and cortical myoclonus. Electroencephalogr Clin Neurophysiol. 1998;109:70–72. doi: 10.1016/s0924-980x(97)00062-3. [DOI] [PubMed] [Google Scholar]
- 108.Shimizu T, Maehara T, Hino T, et al. Effect of multiple subpial transection on motor cortical excitability in cortical dysgenesis. Brain. 2001;124:1336–1349. doi: 10.1093/brain/124.7.1336. [DOI] [PubMed] [Google Scholar]
- 109.Manganotti P, Zanette G. Contribution of motor cortex in generation of evoked spikes in patients with benign rolandic epilepsy. Clin Neurophysiol. 2000;111:964–974. doi: 10.1016/s1388-2457(00)00268-6. [DOI] [PubMed] [Google Scholar]
- 110.Manganotti P, Tamburin S, Zanette G, et al. Hyperexcitable cortical responses in progressive myoclonic epilepsy: a TMS study. Neurology. 2001;57:1793–1799. doi: 10.1212/wnl.57.10.1793. [DOI] [PubMed] [Google Scholar]
- 111.Valzania F, Strafella AP, Tropeani A, et al. Facilitation of rhythmic events in progressive myoclonus epilepsy: a transcranial magnetic stimulation study. Clin Neurophysiol. 1999;110:152–157. doi: 10.1016/s0013-4694(98)00115-1. [DOI] [PubMed] [Google Scholar]
- 112.Brodtmann A, Macdonell RA, Gilligan AK, et al. Cortical excitability and recovery curve analysis in generalized epilepsy. Neurology. 1999;53:1347–1349. doi: 10.1212/wnl.53.6.1347. [DOI] [PubMed] [Google Scholar]
- 113.Macdonell RA, King MA, Newton MR, et al. Prolonged cortical silent period after transcranial magnetic stimulation in generalized epilepsy. Neurology. 2001;57:706–708. doi: 10.1212/wnl.57.4.706. [DOI] [PubMed] [Google Scholar]
- 114.Cincotta M, Borgheresi A, Lori S, et al. Interictal inhibitory mechanisms in patients with cryptogenic motor cortex epilepsy: a study of the silent period following transcranial magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1998;107:1–7. doi: 10.1016/s0013-4694(98)00035-2. [DOI] [PubMed] [Google Scholar]
- 115.Ertas NK, Gul G, Altunhalka A, et al. Cortical silent period following transcranial magnetic stimulation in epileptic patients. Epileptic Disord. 2000;2:137–140. [PubMed] [Google Scholar]
- 116.Di Lazzaro V, Restuccia D, Servidei S, et al. Functional involvement of cerebral cortex in Duchenne muscular dystrophy. Muscle Nerve. 1998;21:662–664. doi: 10.1002/(sici)1097-4598(199805)21:5<662::aid-mus18>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 117.Roricht S, Meyer BU, Irlbacher K, et al. Impairment of callosal and corticospinal system function in adolescents with early-treated phenylketonuria: a transcranial magnetic stimulation study. J Neurol. 1999;246:21–30. doi: 10.1007/s004150050301. [DOI] [PubMed] [Google Scholar]
- 118.Moll GH, Heinrich H, Rothenberger A. Transcranial magnetic stimulation in child and adolescent psychiatry: excitability of the motor system in tic disorders and/or attention deficit hyperactivity disorders. Z Kinder Jugendpsychiatr Psychother. 2001;29:312–323. doi: 10.1024//1422-4917.29.4.312. [DOI] [PubMed] [Google Scholar]
- 119.Moll GH, Heinrich H, Trott GE, et al. Children with comorbid attention-deficit-hyperactivity disorder and tic disorder: evidence for additive inhibitory deficits within the motor system. Ann Neurol. 2001;49:393–396. [PubMed] [Google Scholar]
- 120.Moll GH, Heinrich H, Trott G, et al. Deficient intracortical inhibition in drug-naive children with attention-deficit hyperactivity disorder is enhanced by methylphenidate. Neurosci Lett. 2000;284:121–125. doi: 10.1016/s0304-3940(00)00980-0. [DOI] [PubMed] [Google Scholar]
- 121.Ziemann U, Paulus W, Rothenberger A. Decreased motor inhibition in Tourette’s disorder: evidence from transcranial magnetic stimulation. Am J Psychiatry. 1997;154:1277–1284. doi: 10.1176/ajp.154.9.1277. [DOI] [PubMed] [Google Scholar]
- 122.Moll GH, Heinrich H, Rothenberger A. Methylphenidate and intracortical excitability: opposite effects in healthy subjects and attention-deficit hyperactivity disorder. Acta Psychiatr Scand. 2003;107:69–72. doi: 10.1034/j.1600-0447.2003.02114.x. [DOI] [PubMed] [Google Scholar]
- 123.Moll GH, Wischer S, Heinrich H, et al. Deficient motor control in children with tic disorder: evidence from transcranial magnetic stimulation. Neurosci Lett. 1999;272:37–40. doi: 10.1016/s0304-3940(99)00575-3. [DOI] [PubMed] [Google Scholar]
- 124.Garvey MA, Barker CA, Bartko JJ, et al. The ipsilateral silent period in boys with attention-deficit/hyperactivity disorder. Clin Neurophysiol. 2005;116:1889–1896. doi: 10.1016/j.clinph.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 125.Hallett M. Functional reorganization after lesions of the human brain: studies with transcranial magnetic stimulation. Rev Neurol (Paris) 2001;157:822–826. [PubMed] [Google Scholar]
- 126.Seyal M, Ro T, Rafal R. Increased sensitivity to ipsilateral cutaneous stimuli following transcranial magnetic stimulation of the parietal lobe. Ann Neurol. 1995;38:264–267. doi: 10.1002/ana.410380221. [DOI] [PubMed] [Google Scholar]
- 127.Oliveri M, Rossini PM, Traversa R, et al. Left frontal transcranial magnetic stimulation reduces contralesional extinction in patients with unilateral right brain damage. Brain. 1999;122:1731–1739. doi: 10.1093/brain/122.9.1731. [DOI] [PubMed] [Google Scholar]
- 128.Oliveri M, Rossini PM, Filippi MM, et al. Time-dependent activation of parieto-frontal networks for directing attention to tactile space. A study with paired transcranial magnetic stimulation pulses in right-brain-damaged patients with extinction. Brain. 2000;123:1939–1947. doi: 10.1093/brain/123.9.1939. [DOI] [PubMed] [Google Scholar]
- 129.Mimura M, Kato M, Kato M, et al. Prospective and retrospective studies of recovery in aphasia. Changes in cerebral blood flow and language functions. Brain. 1998;121:2083–2094. doi: 10.1093/brain/121.11.2083. [DOI] [PubMed] [Google Scholar]
- 130.Naeser MA, Martin PI, Nicholas M, et al. Improved picture naming in chronic aphasia after TMS to part of right Broca’s area: an open-protocol study. Brain Lang. 2005;93:95–105. doi: 10.1016/j.bandl.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 131.Naeser MA, Martin PI, Nicholas M, et al. Improved naming after TMS treatments in a chronic, global aphasia patient: case report. Neurocase. 2005;11:182–193. doi: 10.1080/13554790590944663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Walter G, Tormos JM, Israel JA, et al. Transcranial magnetic stimulation in young persons: a review of known cases. J Child Adolesc Psychopharmacol. 2001;11:69–75. doi: 10.1089/104454601750143483. [DOI] [PubMed] [Google Scholar]
- 133.Hufnagel A, Elger CE. Induction of seizures by transcranial magnetic stimulation in epileptic patients. J Neurol. 1991;238:109–110. doi: 10.1007/BF00315692. [DOI] [PubMed] [Google Scholar]
- 134.Akamatsu N, Fueta Y, Endo Y, et al. Decreased susceptibility to pentylenetetrazol-induced seizures after low-frequency transcranial magnetic stimulation in rats. Neurosci Lett. 2001;310:153–156. doi: 10.1016/s0304-3940(01)02116-4. [DOI] [PubMed] [Google Scholar]
- 135.Steinhoff BJ, Stodieck SR, Zivcec Z, et al. Transcranial magnetic stimulation (TMS) of the brain in patients with mesiotemporal epileptic foci. Clin Electroencephalogr. 1993;24:1–5. doi: 10.1177/155005949302400103. [DOI] [PubMed] [Google Scholar]
- 136.Graff-Guerrero A, Gonzales-Olvera J, Ruiz-Garcia M, et al. rTMS reduces focal brain hyperperfusion in two patients with EPC. Acta Neurol Scand. 2004;109:290–296. doi: 10.1046/j.1600-0404.2003.00222.x. [DOI] [PubMed] [Google Scholar]
- 137.Theodore WH, Hunter K, Chen R, et al. Transcranial magnetic stimulation for the treatment of seizures: a controlled study. Neurology. 2002;59:560–562. doi: 10.1212/wnl.59.4.560. [DOI] [PubMed] [Google Scholar]
- 138.Fregni F, Thome-Souza S, Bermpohl F, et al. Antiepileptic effects of repetitive transcranial magnetic stimulation in patients with cortical malformations: an EEG and clinical study. Stereotact Funct Neurosurg. 2005;83:57–62. doi: 10.1159/000086674. [DOI] [PubMed] [Google Scholar]
- 139.Fregni F, Otachi PT, Do Valle A, et al. A randomized clinical trial of repetitive transcranial magnetic stimulation in patients with refractory epilepsy. Ann Neurol. 2006;60:447–455. doi: 10.1002/ana.20950. [DOI] [PubMed] [Google Scholar]
- 140.Joo EY, Han SJ, Chung SH, et al. Antiepileptic effects of low-frequency repetitive transcranial magnetic stimulation by different stimulation durations and locations. Clin Neurophysiol. 2007;188:702–708. doi: 10.1016/j.clinph.2006.11.008. [DOI] [PubMed] [Google Scholar]
- 141.Pugh KR, Mencl WE, Jenner AR, et al. Neurobiological studies of reading and reading disability. J Commun Disord. 2001;34:479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- 142.Simos PG, Fletcher JM, Foorman BR, et al. Brain activation profiles during the early stages of reading acquisition. J Child Neurol. 2002;17:159–163. doi: 10.1177/088307380201700301. [DOI] [PubMed] [Google Scholar]
- 143.Triggs WJ, Kirshner HS. Improving brain function with transcranial magnetic stimulation? Neurology. 2001;56:429–430. doi: 10.1212/wnl.56.4.429. [DOI] [PubMed] [Google Scholar]
- 144.Evers S, Bockermann I, Nyhuis PW. The impact of transcranial magnetic stimulation on cognitive processing: an event-related potential study. Neuroreport. 2001;12:2915–2918. doi: 10.1097/00001756-200109170-00032. [DOI] [PubMed] [Google Scholar]
- 145.Sparing R, Mottaghy FM, Hungs M, et al. Repetitive transcranial magnetic stimulation effects on language function depend on the stimulation parameters. J Clin Neurophysiol. 2001;18:326–330. doi: 10.1097/00004691-200107000-00004. [DOI] [PubMed] [Google Scholar]
- 146.Topper R, Mottaghy FM, Brugmann M, et al. Facilitation of picture naming by focal transcranial magnetic stimulation of Wernicke’s area. Exp Brain Res. 1998;121:371–378. doi: 10.1007/s002210050471. [DOI] [PubMed] [Google Scholar]
- 147.Aimonetti JM, Nielsen JB. Cortical excitability and motor task in man: an investigation of the wrist extensor motor area. Exp Brain Res. 2002;143:431–439. doi: 10.1007/s00221-002-1010-3. [DOI] [PubMed] [Google Scholar]
- 148.Counter SA. Neurobiological effects of extensive transcranial electromagnetic stimulation in an animal model. Electroencephalogr Clin Neurophysiol. 1993;89:341–348. doi: 10.1016/0168-5597(93)90074-y. [DOI] [PubMed] [Google Scholar]
- 149.Gates JR, Dhuna A, Pascual-Leone A. Lack of pathologic changes in human temporal lobes after transcranial magnetic stimulation. Epilepsia. 1992;33:504–508. doi: 10.1111/j.1528-1157.1992.tb01700.x. [DOI] [PubMed] [Google Scholar]
- 150.Sgro JA, Ghatak NR, Stanton PC, et al. Repetitive high magnetic field stimulation: the effect upon rat brain. Electroencephalogr Clin Neurophysiol Suppl. 1991;43:180–185. [PubMed] [Google Scholar]
- 151.Wassermann EM, Grafman J, Berry C, et al. Use and safety of a new repetitive transcranial magnetic stimulator. Electroencephalogr Clin Neurophysiol. 1996;101:412–417. [PubMed] [Google Scholar]
- 152.Anand S, Hotson J. Transcranial magnetic stimulation: neurophysiological applications and safety. Brain Cogn. 2002;50:366–386. doi: 10.1016/s0278-2626(02)00512-2. [DOI] [PubMed] [Google Scholar]
- 153.Pascual-Leone A, Cohen LG, Shotland LI, et al. No evidence of hearing loss in humans due to transcranial magnetic stimulation. Neurology. 1992;42:647–651. doi: 10.1212/wnl.42.3.647. [DOI] [PubMed] [Google Scholar]
- 154.Wassermann EM. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the international workshop on the safety of repetitive transcranial magnetic stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1998;108:1–16. doi: 10.1016/s0168-5597(97)00096-8. [DOI] [PubMed] [Google Scholar]
- 155.Collado-Corona MA, Mora-Magana I, Cordero GL, et al. Transcranial magnetic stimulation and acoustic trauma or hearing loss in children. Neurol Res. 2001;23:343–346. doi: 10.1179/016164101101198532. [DOI] [PubMed] [Google Scholar]