Abstract
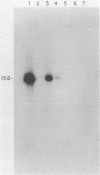
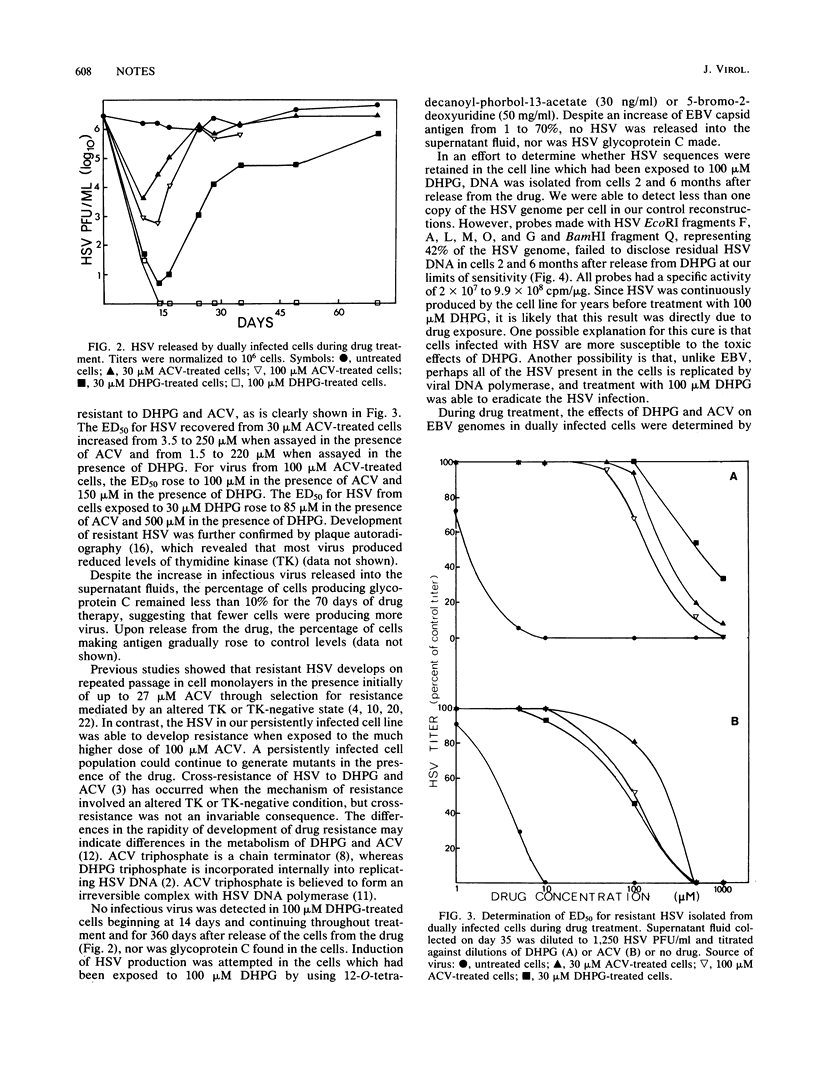
We investigated the effects of acyclovir and 9-(1,3-dihydroxy-2-propoxymethyl)guanine (DHPG) on a lymphoblastoid cell line dually infected with Epstein-Barr virus and herpes simplex virus (HSV) type 1. The numbers of Epstein-Barr virus genomes were reduced during 70 days of treatment with either drug. Both drugs suppressed HSV replication in a dose-related manner. In the continued presence of the drugs, HSV developed resistance, rapidly to acyclovir and much more slowly to 30 microM DHPG. Analysis of HSV glycoprotein C production and viral DNA showed that treatment with 100 microM DHPG eliminated HSV production, curing the cell line of HSV persistent infection.
Full text
PDF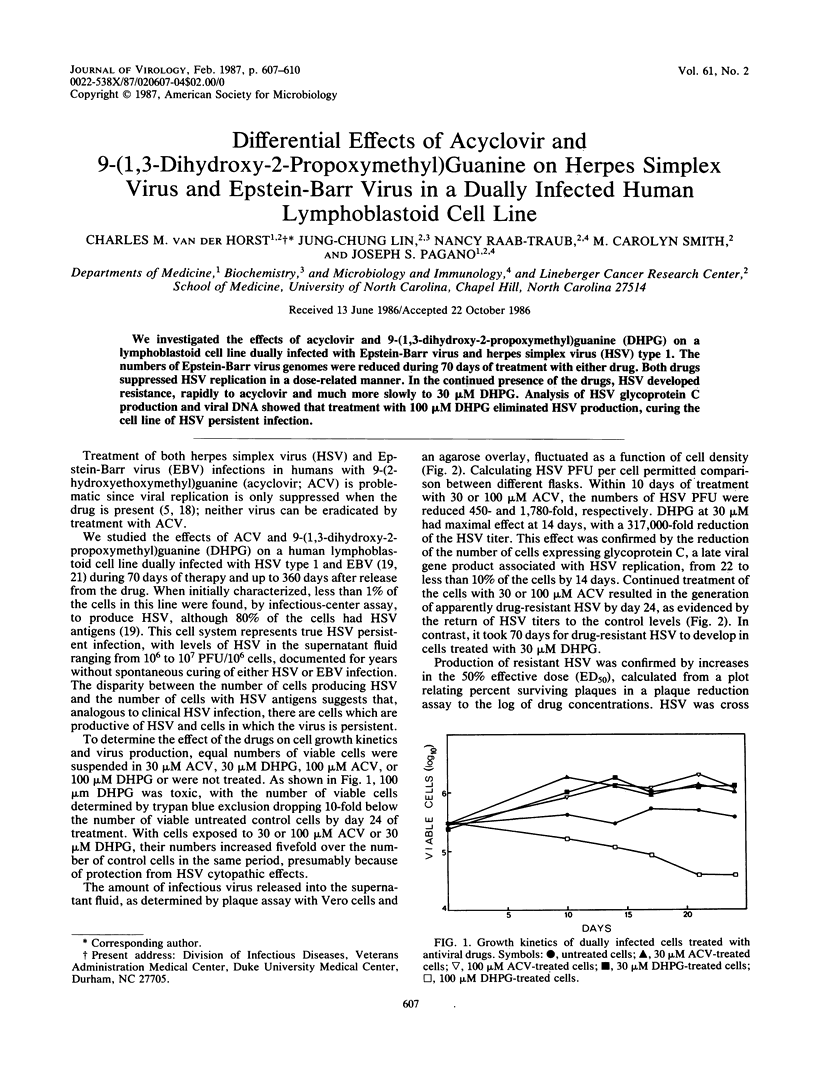


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baer R., Bankier A. T., Biggin M. D., Deininger P. L., Farrell P. J., Gibson T. J., Hatfull G., Hudson G. S., Satchwell S. C., Séguin C. DNA sequence and expression of the B95-8 Epstein-Barr virus genome. Nature. 1984 Jul 19;310(5974):207–211. doi: 10.1038/310207a0. [DOI] [PubMed] [Google Scholar]
- Chen S. T., Estes J. E., Huang E. S., Pagano J. S. Epstein-Barr virus-associated thymidine kinase. J Virol. 1978 Apr;26(1):203–208. doi: 10.1128/jvi.26.1.203-208.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y. C., Grill S. P., Dutschman G. E., Nakayama K., Bastow K. F. Metabolism of 9-(1,3-dihydroxy-2-propoxymethyl)guanine, a new anti-herpes virus compound, in herpes simplex virus-infected cells. J Biol Chem. 1983 Oct 25;258(20):12460–12464. [PubMed] [Google Scholar]
- Cheng Y. C., Huang E. S., Lin J. C., Mar E. C., Pagano J. S., Dutschman G. E., Grill S. P. Unique spectrum of activity of 9-[(1,3-dihydroxy-2-propoxy)methyl]-guanine against herpesviruses in vitro and its mode of action against herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1983 May;80(9):2767–2770. doi: 10.1073/pnas.80.9.2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D. M., Schaffer P. A. Two distinct loci confer resistance to acycloguanosine in herpes simplex virus type 1. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2265–2269. doi: 10.1073/pnas.77.4.2265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crumpacker C. S., Schnipper L. E., Marlowe S. I., Kowalsky P. N., Hershey B. J., Levin M. J. Resistance to antiviral drugs of herpes simplex virus isolated from a patient treated with acyclovir. N Engl J Med. 1982 Feb 11;306(6):343–346. doi: 10.1056/NEJM198202113060606. [DOI] [PubMed] [Google Scholar]
- Datta A. K., Colby B. M., Shaw J. E., Pagano J. S. Acyclovir inhibition of Epstein-Barr virus replication. Proc Natl Acad Sci U S A. 1980 Sep;77(9):5163–5166. doi: 10.1073/pnas.77.9.5163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elion G. B., Furman P. A., Fyfe J. A., de Miranda P., Beauchamp L., Schaeffer H. J. Selectivity of action of an antiherpetic agent, 9-(2-hydroxyethoxymethyl) guanine. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5716–5720. doi: 10.1073/pnas.74.12.5716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field A. K., Davies M. E., DeWitt C., Perry H. C., Liou R., Germershausen J., Karkas J. D., Ashton W. T., Johnston D. B., Tolman R. L. 9-([2-hydroxy-1-(hydroxymethyl)ethoxy]methyl)guanine: a selective inhibitor of herpes group virus replication. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4139–4143. doi: 10.1073/pnas.80.13.4139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field H. J., Darby G. Pathogenicity in mice of strains of herpes simplex virus which are resistant to acyclovir in vitro and in vivo. Antimicrob Agents Chemother. 1980 Feb;17(2):209–216. doi: 10.1128/aac.17.2.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furman P. A., St Clair M. H., Spector T. Acyclovir triphosphate is a suicide inactivator of the herpes simplex virus DNA polymerase. J Biol Chem. 1984 Aug 10;259(15):9575–9579. [PubMed] [Google Scholar]
- Lin J. C., Smith M. C., Cheng Y. C., Pagano J. S. Epstein-Barr virus: inhibition of replication by three new drugs. Science. 1983 Aug 5;221(4610):578–579. doi: 10.1126/science.6306771. [DOI] [PubMed] [Google Scholar]
- Lin J. C., Smith M. C., Pagano J. S. Activation of latent Epstein-Barr virus genomes: selective stimulation of synthesis of chromosomal proteins by a tumor promoter. J Virol. 1983 Mar;45(3):985–991. doi: 10.1128/jvi.45.3.985-991.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin J. C., Smith M. C., Pagano J. S. Prolonged inhibitory effect of 9-(1,3-dihydroxy-2-propoxymethyl)guanine against replication of Epstein-Barr virus. J Virol. 1984 Apr;50(1):50–55. doi: 10.1128/jvi.50.1.50-55.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin J. L., Ellis M. N., Keller P. M., Biron K. K., Lehrman S. N., Barry D. W., Furman P. A. Plaque autoradiography assay for the detection and quantitation of thymidine kinase-deficient and thymidine kinase-altered mutants of herpes simplex virus in clinical isolates. Antimicrob Agents Chemother. 1985 Aug;28(2):181–187. doi: 10.1128/aac.28.2.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagano J. S., Datta A. K. Perspectives on interactions of acyclovir with Epstein-Barr and other herpes viruses. Am J Med. 1982 Jul 20;73(1A):18–26. doi: 10.1016/0002-9343(82)90057-2. [DOI] [PubMed] [Google Scholar]
- Pagano J. S., Sixbey J. W., Lin J. C. Acyclovir and Epstein-Barr virus infection. J Antimicrob Chemother. 1983 Sep;12 (Suppl B):113–121. doi: 10.1093/jac/12.suppl_b.113. [DOI] [PubMed] [Google Scholar]
- Robey W. G., Graham B. J., Harris C. L., Madden M. J., Pearson G. R., Vande Woude G. F. Persistent herpes simplex virus infections established in two Burkitt lymphoma derived cell lines. J Gen Virol. 1976 Jul;32(1):51–62. doi: 10.1099/0022-1317-32-1-51. [DOI] [PubMed] [Google Scholar]
- Schnipper L. E., Crumpacker C. S. Resistance of herpes simplex virus to acycloguanosine: role of viral thymidine kinase and DNA polymerase loci. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2270–2273. doi: 10.1073/pnas.77.4.2270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw J. E., Colby B. M., Leong J., Bolling T. J. Effect of acyclovir on herpes simplex virus replication in a persistently infected human lymphoblastoid cell line (P3HR-1). Am J Med. 1982 Jul 20;73(1A):72–76. doi: 10.1016/0002-9343(82)90067-5. [DOI] [PubMed] [Google Scholar]
- Smith K. O., Kennell W. L., Poirier R. H., Lynd F. T. In vitro and in vivo resistance of herpes simplex virus to 9-(2-hydroxyethoxymethyl)guanine (acycloguanosine). Antimicrob Agents Chemother. 1980 Feb;17(2):144–150. doi: 10.1128/aac.17.2.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcombe T., Clough W. Epstein-Barr virus induces a unique pyrimidine deoxynucleoside kinase activity in superinfected and virus-producer B cell lines. Biochemistry. 1985 Apr 9;24(8):2027–2033. doi: 10.1021/bi00329a034. [DOI] [PubMed] [Google Scholar]
- de Turenne-Tessier M., Ooka T., de The G., Daillie J. Characterization of an Epstein-Barr virus-induced thymidine kinase. J Virol. 1986 Mar;57(3):1105–1112. doi: 10.1128/jvi.57.3.1105-1112.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]