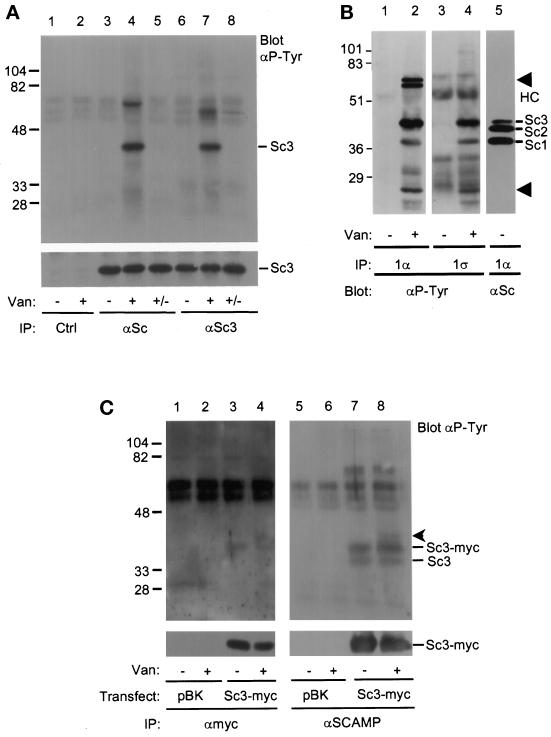
Figure 1.
SCAMP3 is tyrosine phosphorylated in vanadate-treated CHO cells. (A) CHO cells were either untreated (lanes 1, 3, and 6) or treated (lanes 2, 4, and 7) with 5 μM pervanadate for 20 min and immunoprecipitated with anti-SCAMP (7C12, lanes 3–5), anti-SCAMP3 (3γ, lanes 6–8), or a control antibody (9E10, lanes 1 and 2). Two samples (lanes 5 and 8, +/−) were washed in vanadate-free media and further incubated for 1 h. Immunoprecipitates were Western blotted with anti-phosphotyrosine (αP-Tyr, upper panel) or anti-SCAMP3 (3γ, lower panel) and visualized using ECL. The corresponding 7C12 blot demonstrated that bands with the mobility of SCAMP1 and SCAMP3, but not SCAMP2, were phosphorylated on tyrosine (our unpublished observations). (B) Untreated (lanes 1, 3, and 5) or vanadate-treated (lanes 2 and 4) CHO cells were lysed in CHAPS buffer and immunoprecipitated with anti-SCAMP1 antibodies 1α (lanes 1, 2, and 5) or 1ς (lanes 3 and 4). Samples were resolved by SDS-PAGE, transferred to nitrocellulose, and Western blotted with anti-phosphotyrosine antibody (αP-Tyr, lanes 1–4) or with anti-SCAMP (7C12, lane 5). The positions of SCAMPs 1, 2, and 3 are indicated (Sc1, Sc2, Sc3), as well as the position of several unidentified phosphotyrosine proteins (lanes 2 and 4, arrowheads) and the IgG heavy chain (HC). (C) Tyrosine phosphorylation of endogenous SCAMP3 and transiently overexpressed SCAMP3-myc. 293T cells were transfected with expression vector pBK alone (lanes 1 and 2) or with human SCAMP3-myc/pBK (lanes 3 and 4), lysed in CHAPS buffer 48 h post-transfection, and immunoprecipitated with anti-myc antibody (9E10, lanes 1–4). The supernatants from samples 1–4 were then immunoprecipitated with anti-SCAMP antibody (7C12, lanes 5–8). Immunoprecipitates were Western blotted with anti-phosphotyrosine antibody (upper panels) or anti-myc (lower panel) and visualized by ECL. An unknown protein of ∼40 kDa coimmunoprecipitates with SCAMP3-myc (lanes 4 and 8, arrowhead).