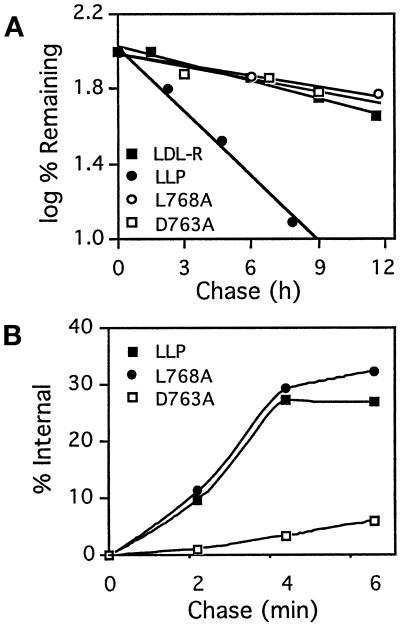
Figure 2.
Turnover and internalization of LLP chimeric proteins containing the native or mutant cytoplasmic domain of P-selectin. (A) Turnover of LLP constructs. CHO cells expressing the indicated LLP constructs were labeled with biotin at 0°C and then recultured at 37°C for the indicated times before cell lysis and immunoprecipitation of the chimeric proteins. Immunoprecipitates were resolved by SDS-PAGE, transferred to nitrocellulose, and probed with 125I-streptavidin. Radioactivity in the bands was quantitated using the PhosphorImager. Data for native LDL receptor (LDL-R) are from Green et al. (1994). (B) Internalization of LLP constructs. Cells were labeled with disulfide-linked biotin at 0°C, warmed to 37°C for the indicated intervals, and then incubated at 0°C with glutathione to reduce exposed disulfide bonds. Proteins were immunoprecipitated from detergent lysates, separated on nonreducing SDS-PAGE, and transferred to nitrocellulose. Biotin was detected with 125I-streptavidin and quantitated by PhosphorImager analysis. Background, defined as the signal obtained from cells that were labeled but not warmed to 37°C before glutathione treatment (0 chase), was 3–5% of the total label (no glutathione treatment) and was subtracted from all data points.