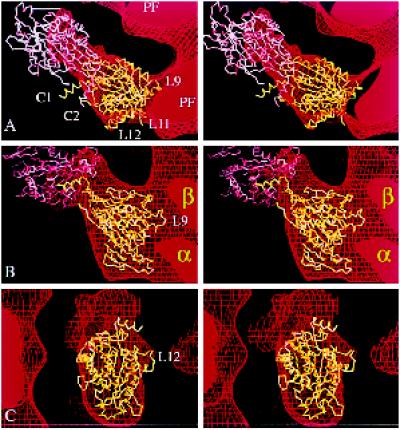
Figure 6.
Fitting the atomic structure of kinesin into our kinesin·ADP map. Stereo views of two copies of the α-carbon backbone of the kinesin monomer (Sack et al., 1997) oriented inside part of the EM map (in Figure 3B) that shows tubulin protofilaments (PF) decorated with dimeric kinesin·ADP. (a) A top view, seen from the microtubule plus end. (b) A side view. (c) The view, at 90° to both a and b, showing the attached head from outside the microtubule. The map is represented as a surface net (red lines). One kinesin molecule (shown in yellow) occupies the attached head density; another (in pink) is positioned in the tethered head density. The relative orientation of the pink and yellow molecules mimics, as closely as possible, the asymmetric arrangement of heads in crystals of dimeric kinesin (Kozielski et al., 1997); thus, loop L10 of the attached head B contacts loop L8 of the tethered head A. A small distortion of each C-terminal helix (C1 and C2) would be needed to bring them together to form a coiled coil; this rod would then point almost directly away from the microtubule.