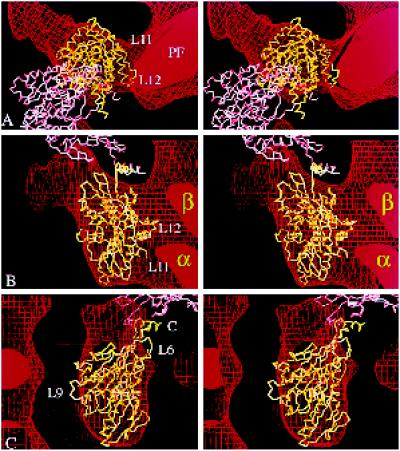
Figure 7.
Alternative docking of kinesin into our kinesin·ADP map. (a–c) Stereo views showing two copies of the α-carbon backbone of monomeric kinesin oriented inside the same EM map of kinesin·ADP shown in Figure 6 but docked in the orientation favored by Hoenger et al. (1998). The directly bound head (yellow) fills the outer boundaries almost as well as in Figure 6, but the top (a) and side (c) views fit less accurately, and in the side view (b), the complementarity of kinesin’s inside surface with the tubulin subunits (α and β) is less good. A second molecule (pink) arranged as in the crystallographic structure is completely out of the EM density, as can be seen in a. The coiled coil would point obliquely into the microtubule surface. C, C terminus; PF, protofilament.