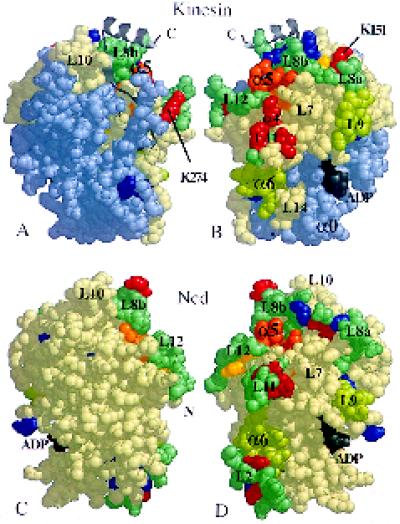
Figure 9.
Exposed and protected surfaces of the bound motor domains. Space-filling images of monomeric kinesin and Ncd, showing the positions of residues that appear to be important. (A and C) The views from directly outside a decorated microtubule for heads bound in the orientation shown in Figure 6; the head (B and D) has been rotated by 180° to show the surface closest to tubulin. Cleavable residues are either protected from proteolysis by microtubules (red), including lysines K151 and K274, or always exposed (blue) (Alonso et al., 1998); yellow residues are cut differentially in ADP versus AMP·PNP, in the absence of microtubules. Alanine scanning (Woehlke et al., 1997) of kinesin suggests that the orange arginine and lysine residues (R280, K283, and R286—corresponding to R278, K281, and R284 in human kinesin, respectively) in the L12/α5 region are important for the binding of microtubules. Some predicted tubulin-binding loops (L7, L8, L11, and L12 [Kull et al., 1996]) are shown in blue-green. Helices α4 and α6 and loops L7 and L9 also appear to be exposed to tubulin. The light-blue part of kinesin represents residues 1–130 (including α0), which can be removed without loss of microtubule binding (Yang et al., 1989). N and C, chain termini.