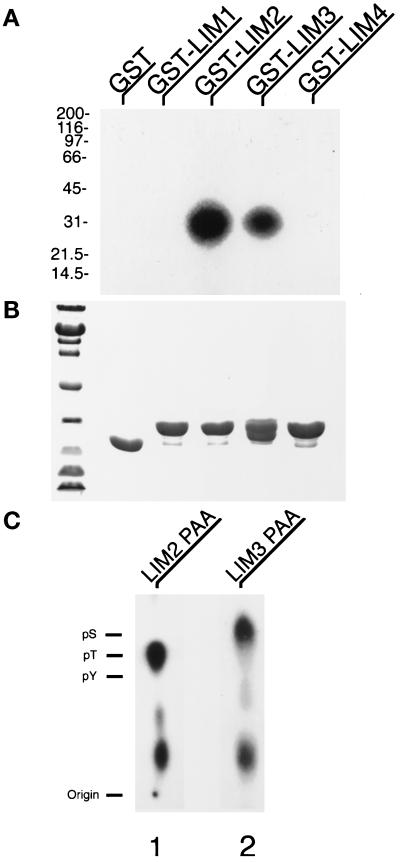
Figure 2.
Phosphorylation of paxillin LIM domains 2 and 3 on threonine and serine. (A) GST fusion proteins comprising the four individual LIM domains of paxillin were incubated with avian smooth muscle lysates and washed free of unbound protein, followed by protein kinase assay as described in MATERIALS AND METHODS. Lane 1, GST alone; lane 2, GST-LIM1; lane 3, GST-LIM2; lane 4, GST-LIM3; and lane 5, GST-LIM4. Only GST-LIM2 and GST-LIM3 specifically precipitated and were phosphorylated by protein kinases. (B) Coomassie brilliant blue staining of the GST precipitation kinase assay SDS-polyacrylamide gel to show equivalent loading of the fusion proteins. (C) PAA was performed on the phosphorylated GST-LIM2 and GST-LIM3 fusion proteins. Comigration of ninhydrin-stained phosphoamino acid standards revealed that the phosphorylation of LIM2 was restricted to the amino acid threonine and paxillin LIM3 on serine. Lower spots are partial hydrolysis products.