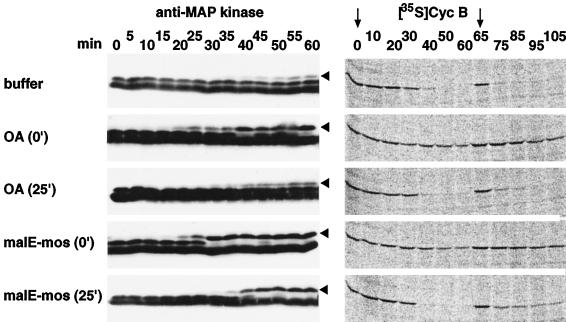
Figure 8.
Activation of MAP kinase by OA and malE-mos correlates with the inhibition of cyclin B degradation. The electrophoretic mobility shift of MAP kinase that accompanies its activation and the stability of 35S-labeled cyclin B were examined in extracts entering mitosis, induced by addition of Δ90 at time zero. The extracts contained either 1 μM OA (added at time 0 or after 25 min), 20 μg/ml malE-mos (added at time 0 or after 25 min), or DMSO as a control (buffer). 35S-labeled cyclin B, Δ90, and ubiquitin were added a second time after 65 min. This was done to exclude the possibility that addition of OA or malE-mos after 25 min did not prevent cyclin B proteolysis, because the degradation reactions were completed before these reagents could activate MAP kinase. Samples were taken at the indicated time points and analyzed by SDS-PAGE and immunoblotting with Erk2 antibodies (anti-MAP kinase) or phosphorimaging (Cyc B). The slower-migrating bands representing active MAP kinase are indicated by arrowheads. The time points of 35S-labeled cyclin B addition are marked by arrows.