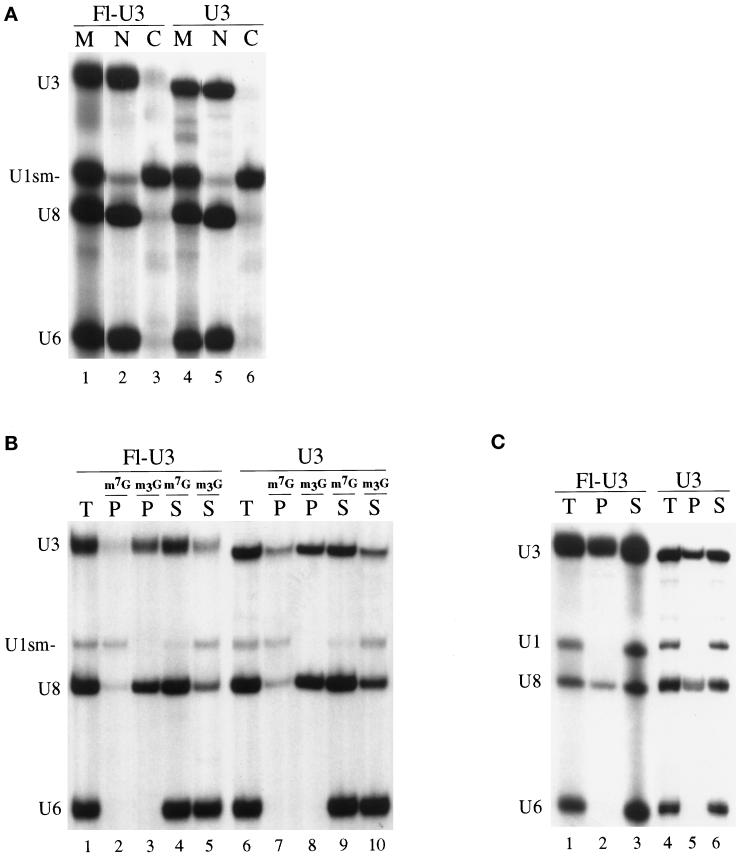
Figure 1.
Nuclear retention, cap hypermethylation, and fibrillarin binding of fluorescein-labeled U3 RNA in Xenopus oocytes. (A) Nucleocytoplasmic distribution of fluorescein-labeled U3 snoRNA (Fl-U3) or nonfluorescein-labeled U3 snoRNA (U3) and U1sm− snRNA, U8 snoRNA, and U6 snRNA after their injection into oocyte nuclei. 32P-labeled, m7G-capped Fl-U3, U3, U1sm−, U8, and U6 were synthesized in vitro, and mixtures of the RNAs were injected into nuclei of Xenopus oocytes. After 8 h of incubation at 18°C, the labeled RNAs present in the nuclear (N) and cytoplasmic (C) fractions of the oocytes were isolated and analyzed by electrophoresis in a denaturing polyacrylamide gel. Lanes 1 and 4 (M) show the RNAs before injection. Lanes 2 and 3 show the distribution of Fl-U3, and lanes 5 and 6 show that of nonfluorescein-labeled U3. (B) Identification of the 5′ cap structure (m7G or m2,2,7G) by immunoprecipitation. The RNAs present in the nucleus 4 h after injection were precipitated using anti-m7G (m7G) (Munns et al., 1982) or anti-m2,2,7G (m3G) (Bringmann et al., 1983) cap antibodies as indicated. The RNAs present in the total sample (T), precipitate (P), and supernatant (S) fractions were separated by gel electrophoresis as described in A. Lanes 1–5 show the precipitation profile of Fl-U3, and lanes 6–10 show that of nonfluorescein-labeled U3. (C) Determination of fibrillarin binding to fluorescein-labeled U3. Immunoprecipitations were performed on nuclear extracts prepared 4 h after injection of RNAs using anti-fibrillarin antibodies (72B9) (Reimer et al., 1987). RNAs present in the precipitate (P), 20% of the total sample (T), and 20% of the supernatant (S) were separated by gel electrophoresis as described in A. Lanes 1–3 show the precipitation profile of Fl-U3, and lanes 4–6 show that of nonfluorescein-labeled U3. Note that the gel electrophoretic mobility of the fluorescein-labeled U3 RNA is reduced relative to that of the unlabeled U3 in A–C.