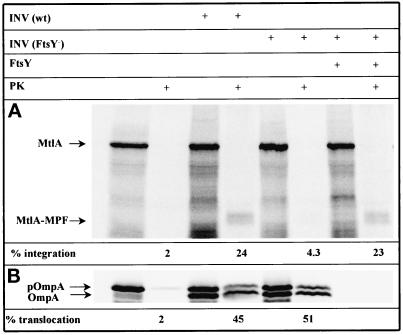
Figure 2.
Depletion of FtsY affects integration of membrane proteins but does not impair translocation of secretory proteins. MtlA and OmpA were synthesized in vitro by coupled transcription/translation of plasmids pMtlII-9, encoding mtlA under the SP6 promoter (A), and pDMB, coding for ompA under the T7 promoter (B), in a reconstituted cell-free translation system from E. coli (for further details see MATERIALS AND METHODS). Cell-free translation products labeled with [35S]methionine were either directly precipitated with trichloroacetic acid or only after incubation with 0.5 mg/ml proteinase K for 20 min at 25°C (PK). Thereafter, proteins were separated by SDS-PAGE (15% acrylamide) and visualized by phosphorimaging. Indicated are the positions of full-length MtlA, the fragment of MtlA resistant toward proteinase K (MtlA-MPF), the precursor (pOmpA), and the signal sequence-free form of OmpA. In vitro syntheses were performed in the presence of the reagents indicated at the top of the figure (INV (wt), wild-type E. coli inner-membrane vesicles; INV (FtsY−), membrane vesicles prepared from the conditional FtsY mutant E. coli N4156 pAra14-FtsY′; FtsY, purified FtsY [20 ng/μl final concentration]). The percentage of integration was calculated after quantitation of the radioactivity of individual protein bands using a Phosphorimager and calculating the ratio between MtlA-MPF and MtlA. The values thus obtained were corrected for the loss of Met residues occurring during cleavage by proteinase K. The percentage of translocation equals the ratio of radioactivity present in the proteinase K-resistant bands of pOmpA and OmpA and that recovered from the corresponding bands before proteolytic digestion (the INV used here usually give rise to some proteinase K-resistant pOmpA representing a fraction of uncleaved yet translocated precursor).