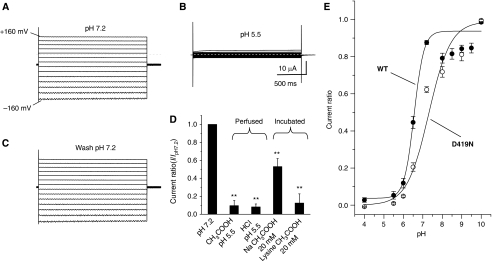
Figure 5.
DEDG/ENaC channel ACD-1 exhibits a strong sensitivity to acidic solutions. (A) Example of currents elicited by voltage steps from −160 to +100 mV from a holding potential of −30 mV in a Xenopus oocyte injected with acd-1 cRNA and exposed to a physiological NaCl saline solution at pH 7.2. (B) The same oocyte was exposed to a solution at pH 5.5, which resulted in the inhibition of ACD-1 currents. (C) When we returned to physiological pH 7.2, ACD-1 currents returned to control levels. (D) ACD-1 currents are equally suppressed by extracellular solutions buffered with acetic acid and HCl and are reduced by incubation with 20 mM extracellular Na acetate and lysine acetate, suggesting that both extracellular and intracellular acidification cause channel inhibition (Thomas, 1984; Zampighi et al, 1988). For incubation with 20 mM Na acetate and 20 mM lysine acetate, we incubated oocytes in the NaCl solution in which we added Na acetate or lysine acetate for 1 h at room temperature. Control oocytes were incubated under the same conditions in NaCl physiological saline. Oocytes were then transferred to control NaCl solution for 15 min prior to recordings. We could not recover currents to control levels after 3 h of wash in Na acetate- and lysine acetate-treated oocytes. This is not unusual and it has been observed by others using this technique for intracellular acidification (Thomas, 1984; Zampighi et al, 1988). n was 8 for each bar. (E) Sensitivity of wild-type ACD-1 and ACD-1(D419N) channels to extracellular pH. Currents were recorded at -160 mV, normalized to maximal currents and best fitted with the Hill's equation (Ki=pH 6.4, n=0.9 for wild-type ACD-1; Ki for ACD-1(D419N) was 7.3). n is 4–27. Data are expressed as mean±s.e. **Indicates P<0.01 by comparison with untreated oocytes by t-test.