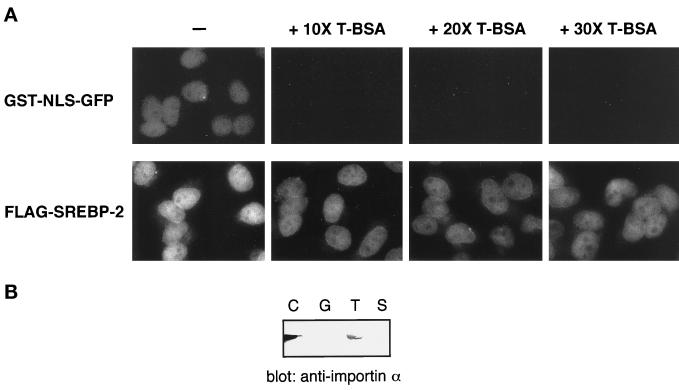
Figure 2.
(A) Basic NLS-containing substrate cannot compete with the nuclear import of SREBP-2 in permeabilized cells. Digitonin-permeabilized cells were incubated with 10 μl of a reaction mixture containing 4 pmol of GST-NLS-GFP or FLAG-SREBP-2 in the absence (−) or presence of 40 pmol (+ 10× T-BSA), 80 pmol (+ 20× T-BSA), or 120 pmol (+ 30× T-BSA) of T-bBSA. All reaction mixtures contained 4 mg/ml cytosol and an ATP regeneration system. After incubation for 20 min at 30°C, the cells were fixed, and the localization of GST-NLS-GFP and FLAG-SREBP-2 was examined by fluorescent microscopy. All photographs for the same substrate were taken with the same exposure time, and the images were obtained by scanning photographic negatives using Adobe (Mountain View, CA) Photoshop version 5.0 under the same conditions. (B) SREBP-2 does not interact with importin α. Cytosol (90 μl) was incubated with GST (G), GST-NLS-GFP (T), or GST-SREBP-2 (S). GST fusion proteins were then captured on glutachione-Sepharose beads. The beads were then washed, and the bound proteins were eluted by boiling in SDS sample buffer. Eluates were separated on 10% SDS-PAGE and analyzed by immunoblotting using anti-mouse importin α polyclonal antibodies. Cytosol (2 μl) was loaded directly onto SDS-PAGE (C).