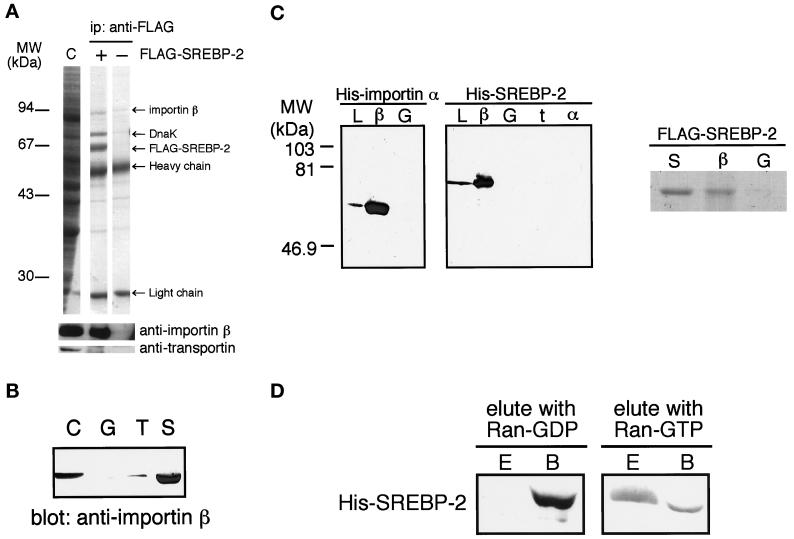
Figure 4.
(A) Importin β is coprecipitated with FLAG-SREBP-2 from cytosol. Cytosol was incubated with (+) or without (−) recombinant partially purified FLAG-SREBP-2 and precipitated with an anti-FLAG M2 antibody. Coprecipitated proteins were separated on 12.5% SDS-PAGE and analyzed by Coomassie blue staining followed by immunoblotting using polyclonal anti-importin β antibodies and monoclonal anti-transportin antibody. Importin β (∼94 kDa) was coprecipitated by an anti-FLAG antibody with FLAG-SREBP-2 (+) but not by antibody alone (−). Note that although E. coli protein DnaK was contaminated with partially purified FLAG-SREBP-2, we verified that it does not affect the results of immunoprecipitation by using purified FLAG-SREBP-2 (our unpublished results). (B) Importin β is pulled down by GST-SREBP-2 from cytosol. Cytosol (90 μl) was pulled down with GST (G), GST-NLS-GFP (T), or GST-SREBP-2 (S) and analyzed as in Figure 2B except for using polyclonal anti-importin β antibodies. Cytosol (2 μl) was loaded directly onto SDS-PAGE (C). (C) SREBP-2 binds directly to importin β. Left, E. coli lysate expressing His-SREBP-2(1–481) or His-importin α was incubated with GST (G), GST-importin β (β), GST-transportin (t), or GST-importin α (α), and the GST-fusion proteins were captured on glutathione-Sepharose beads. The beads were then washed, and the bound proteins were analyzed by 12.5% SDS-PAGE followed by immunoblotting using anti-penta His antibody. E. coli lysate expressing each His-tagged protein was directly loaded onto the gel (L). Right, purified FLAG-SREBP-2 (5 μg) was incubated with 8 μg of GST-importin β (β) or GST (G) in transport buffer for 1.5 h at 4°C, and the GST fusion proteins were then captured on glutathione-Sepharose beads. The beads were then washed with transport buffer, and the bound proteins were eluted by boiling in SDS sample buffer. Half of the eluted proteins were separated on 10% SDS-PAGE and visualized by Coomassie blue staining. Purified FLAG-SREBP-2 (1 μg) was directly loaded onto the gel (S). (D) SREBP-2 is released from importin β by Ran-GTP but not by Ran-GDP. His-SREBP-2(1–481) was bound to GST-importin β and absorbed on glutathione beads as in C. After extensive washing, the beads were incubated with the transport buffer containing either 10 μM Ran-GDP or G19VRan-GTP for 30 min at 4°C and pelleted by centrifugation. Eluates (E) and bound fractions (B) were separated on 10% SDS-PAGE and analyzed by immunoblotting using anti-penta His antibody.