Abstract
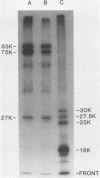
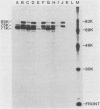
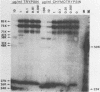
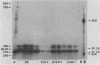
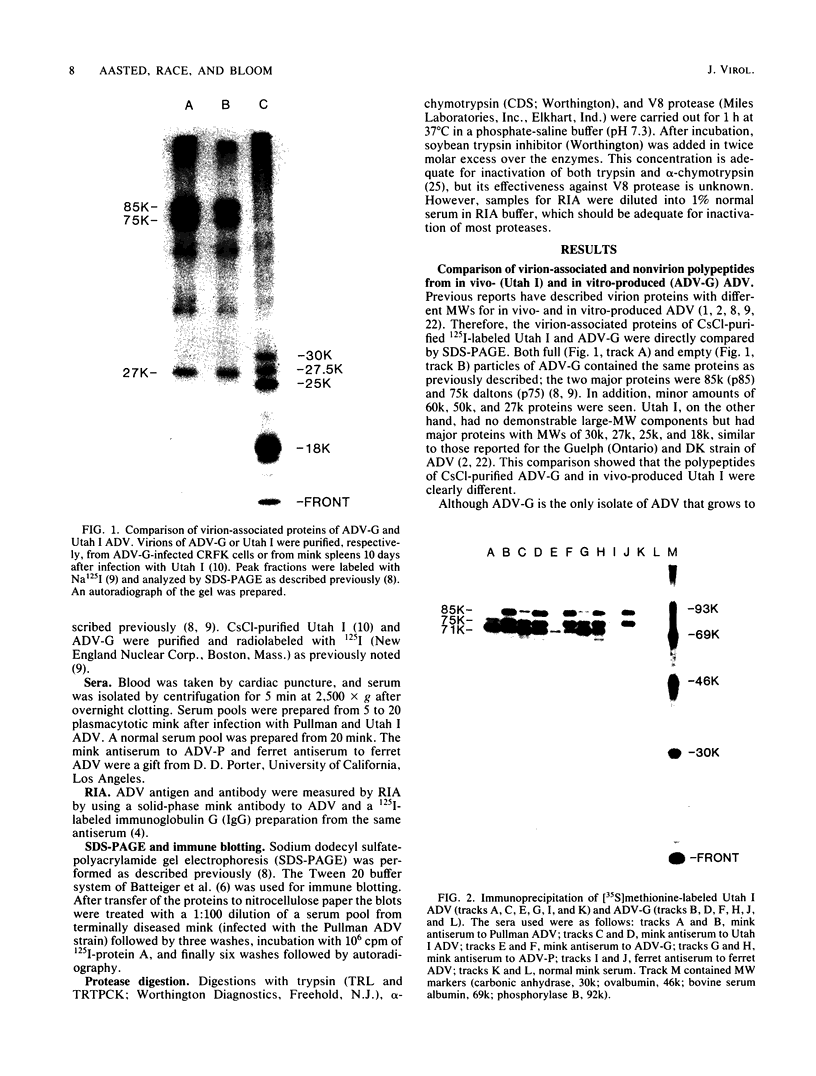
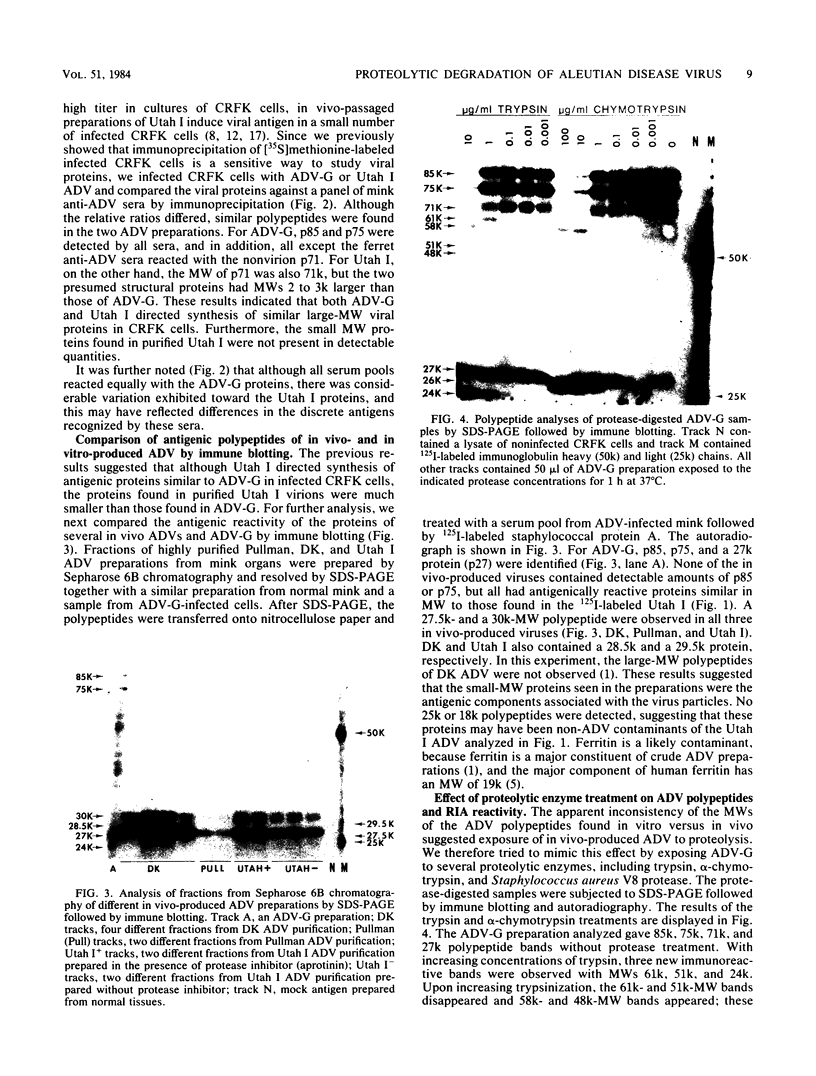
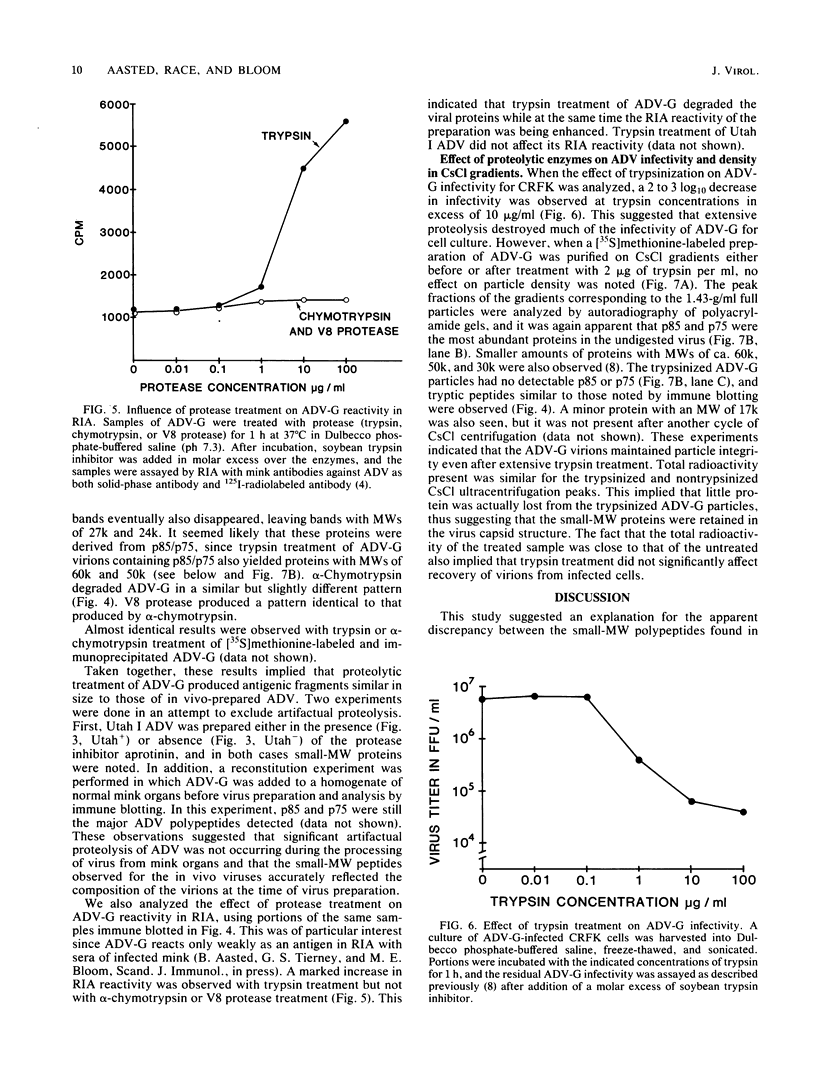
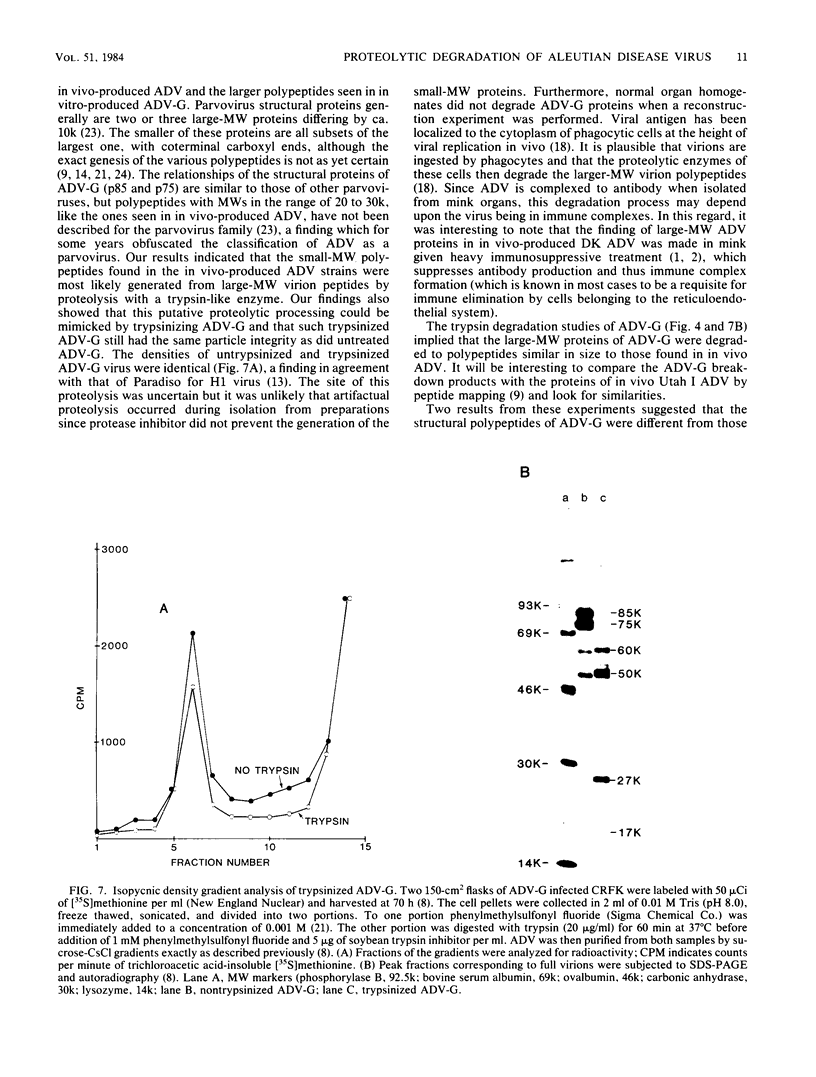
The polypeptides of the highly virulent mink-passaged Utah I and the nonvirulent cell culture-adapted ADV-G strain of Aleutian disease virus (ADV) were compared. When CRFK cells infected with either Utah I or ADV-G were analyzed by immunoprecipitation, both viruses induced proteins with molecular weights characteristic of the ADV-G 85,000 ( 85k )- and 75k-dalton structural proteins (p85 and p75) as well as the 71k -dalton nonvirion protein p71 . However, when Utah I, Pullman ADV, and DK ADV (a Danish isolate of ADV) were purified from infected mink, only polypeptides with molecular weights between 27k and 30k could be identified. In addition, trypsin treatment of ADV-G degraded p85 and p75 to smaller antigenic proteins with molecular weights of 24k and 27k, similar to those found for the virulent in vivo viruses. The effect of proteolytic treatment of ADV was then studied in detail. Purification of Utah I ADV from mink organs in the presence of protease inhibitor did not prevent the appearance of the low-molecular-weight proteins and ADV-G proteins were not degraded upon purification from a homogenate of normal mink organs, suggesting that artifactual proteolysis was not occurring. When a serum pool from terminally diseased mink was analyzed by radioimmunoassay for antibody reactivity against trypsinized and nontrypsinized ADV-G, five times higher reactivity was found for the trypsinized ADV-G than for the nontrypsinized ADV-G, an effect which could not be elicited by chymotrypsin or V8 protease treatment, implying that in vivo-produced ADV was being modulated in vivo by trypsin or a trypsin-like enzyme. Trypsinization was shown not to cause a change in ADV virion density, but to decrease the in vitro infectivity of ADV-G for CRFK cells. These studies suggested that during infection of mink ADV proteins are degraded to highly antigenic smaller polypeptides.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aasted B., Avery B. An easy method for comparing antibody affinities to related antigens. Acta Pathol Microbiol Immunol Scand C. 1983 Feb;91(1):65–67. [PubMed] [Google Scholar]
- Aasted B., Bloom M. E. Sensitive radioimmune assay for measuring Aleutian disease virus antigen and antibody. J Clin Microbiol. 1983 Sep;18(3):637–644. doi: 10.1128/jcm.18.3.637-644.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aasted B. Purification and characterization of Aleutian disease virus. Acta Pathol Microbiol Scand B. 1980 Dec;88(6):323–328. doi: 10.1111/j.1699-0463.1980.tb02650.x. [DOI] [PubMed] [Google Scholar]
- Arosio P., Adelman T. G., Drysdale J. W. On ferritin heterogeneity. Further evidence for heteropolymers. J Biol Chem. 1978 Jun 25;253(12):4451–4458. [PubMed] [Google Scholar]
- Batteiger B., Newhall W. J., 5th, Jones R. B. The use of Tween 20 as a blocking agent in the immunological detection of proteins transferred to nitrocellulose membranes. J Immunol Methods. 1982 Dec 30;55(3):297–307. doi: 10.1016/0022-1759(82)90089-8. [DOI] [PubMed] [Google Scholar]
- Bloom M. E., Mayer L. W., Garon C. F. Characterization of the Aleutian disease virus genome and its intracellular forms. J Virol. 1983 Mar;45(3):977–984. doi: 10.1128/jvi.45.3.977-984.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Characterization of Aleutian disease virus as a parvovirus. J Virol. 1980 Sep;35(3):836–843. doi: 10.1128/jvi.35.3.836-843.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Wolfinbarger J. B. Identification of a nonvirion protein of Aleutian disease virus: mink with Aleutian disease have antibody to both virion and nonvirion proteins. J Virol. 1982 Aug;43(2):608–616. doi: 10.1128/jvi.43.2.608-616.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Bloom M., Hadlow W., Race R. Purification and ultrastructure of Aleutian disease virus of mink. Nature. 1975 Apr 3;254(5499):456–457. doi: 10.1038/254456a0. [DOI] [PubMed] [Google Scholar]
- Eklund C. M., Hadlow W. J., Kennedy R. C., Boyle C. C., Jackson T. A. Aleutian disease of mink: properties of the etiologic agent and the host responses. J Infect Dis. 1968 Dec;118(5):510–526. doi: 10.1093/infdis/118.5.510. [DOI] [PubMed] [Google Scholar]
- Hahn E. C., Ramos L., Kenyon A. J. Expression of Aleutian mink disease antigen in cell culture. Infect Immun. 1977 Jan;15(1):204–211. doi: 10.1128/iai.15.1.204-211.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PORTER D. D., DIXON F. J., LARSEN A. E. METABOLISM AND FUNCTION OF GAMMA GLOBULIN IN ALEUTIAN DISEASE OF MINK. J Exp Med. 1965 Jun 1;121:889–900. doi: 10.1084/jem.121.6.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paradiso P. R. Infectious process of the parvovirus H-1: correlation of protein content, particle density, and viral infectivity. J Virol. 1981 Sep;39(3):800–807. doi: 10.1128/jvi.39.3.800-807.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pintel D., Dadachanji D., Astell C. R., Ward D. C. The genome of minute virus of mice, an autonomous parvovirus, encodes two overlapping transcription units. Nucleic Acids Res. 1983 Feb 25;11(4):1019–1038. doi: 10.1093/nar/11.4.1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Cox N. A., Porter H. G., Suffin S. C. Isolation of Aleutian disease virus of mink in cell culture. Intervirology. 1977;8(3):129–144. doi: 10.1159/000148888. [DOI] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. Aleutian disease of mink. Adv Immunol. 1980;29:261–286. doi: 10.1016/s0065-2776(08)60046-2. [DOI] [PubMed] [Google Scholar]
- Porter D. D., Larsen A. E., Porter H. G. The pathogenesis of Aleutian disease of mink. I. In vivo viral replication and the host antibody response to viral antigen. J Exp Med. 1969 Sep 1;130(3):575–593. doi: 10.1084/jem.130.3.575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter H. G., Porter D. D., Larsen A. E. Aleutian disease in ferrets. Infect Immun. 1982 Apr;36(1):379–386. doi: 10.1128/iai.36.1.379-386.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhode S. L., 3rd, Paradiso P. R. Parvovirus genome: nucleotide sequence of H-1 and mapping of its genes by hybrid-arrested translation. J Virol. 1983 Jan;45(1):173–184. doi: 10.1128/jvi.45.1.173-184.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shahrabadi M. S., Cho H. J., Marusyk R. G. Characterization of the protein and nucleic acid of Aleutian disease virus. J Virol. 1977 Aug;23(2):353–362. doi: 10.1128/jvi.23.2.353-362.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tattersall P., Shatkin A. J., Ward D. C. Sequence homology between the structural polypeptides of minute virus of mice. J Mol Biol. 1977 Apr 25;111(4):375–394. doi: 10.1016/s0022-2836(77)80060-0. [DOI] [PubMed] [Google Scholar]
- WU F. C., LASKOWSKI M. Action of the naturally occurring trypsin inhibitors against chymotrypsins alpha and beta. J Biol Chem. 1955 Apr;213(2):609–619. [PubMed] [Google Scholar]