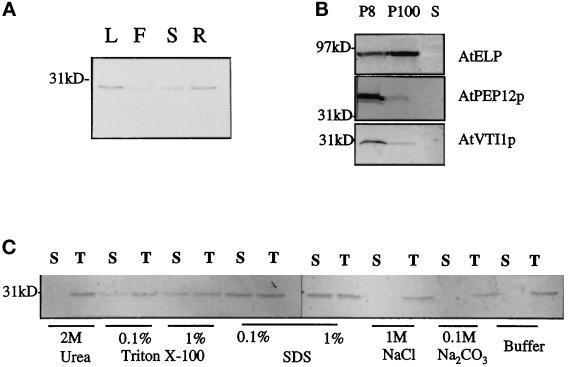
Figure 5.
AtVTI1a is an integral membrane protein. (A) Distribution of AtVTI1a in Arabidopsis organs. Equal amounts of total protein from leaves (L), flowers (F), stems (S), and roots (R) were separated by SDS-PAGE and immunoblotted with guinea pig antiserum against AtVTI1a. Molecular mass is indicated on the left. (B) AtVTI1a fractionates with heavy membranes during differential centrifugation. A postnuclear supernatant from 0.5 g of cultured roots was centrifuged at 8000 × g for 20 min. The pellet (P8) was solubilized in 200 μl of 2× Laemmli loading buffer. The supernatant (S8) was further ultracentrifuged at 100,000 × g for 2 h. The pellet (P100) was solubilized in 200 μl of 2× Laemmli buffer. Equal amounts of the supernatant (S100), P100, and P8 were separated by SDS-PAGE and immunoblotted with anti-AtVTI1a, anti-AtELP, or anti-AtPEP12p antiserum. Molecular mass is indicated on the left. (C) AtVTI1a is an integral membrane protein. Equal amounts of total membranes from Arabidopsis cultured cells were treated with 2 M urea, 0.1 or 1% Triton X-100, 0.1 or 1% SDS, 1 M NaCl, 0.1 M Na2CO3, pH 11, or extraction buffer alone. All treatments were performed at room temperature for 30 min. An aliquot of each treatment was saved as total. Membranes were pelleted by centrifugation at 100,000 × g for 1 h after the treatments. Equal volumes of supernatant or total were separated by SDS-PAGE and immunoblotted with anti-AtVTI1a antiserum. S, supernatant, T, total.