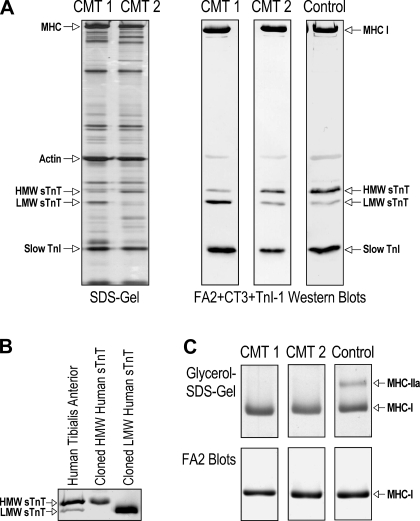
Fig. 1.
Upregulation of the low-molecular-weight (LMW) slow troponin T (sTnT) isoform in muscles of Charcot-Marie-Tooth disease (CMT) type 1 (CMT1) but not CMT type 2 (CMT2) patients. A: represent SDS-PAGE and Western blots of total myofilament protein extracts of skinned single fibers isolated from tibialis anterior muscle biopsies. The silver-stained SDS gel showed similar protein profiles in CMT2 and normal skeletal muscle fibers. In contrast, CMT1 samples showed an increased intensity of an ∼30-kDa protein band. Parallel Western blots using a mixture of monoclonal antibodies (mAb) CT3, TnI-1, and FA2 revealed that CMT1 muscle fibers had a switch of sTnT isoform expression consisting of a significant increase in the LMW sTnT isoform and a decrease in the high-molecular-weight (HMW) sTnT isoform, whereas CMT2 fibers contained mainly the HMW sTnT isoform, which was the same in control fibers. The expression of slow troponin I (TnI) and myosin heavy chain (MHC)-I isoforms was unchanged in CMT1 and CMT2 muscles. B: identities of HMW and LMW sTnT isoforms were confirmed by their gel mobility in CT3 Western blots along with control proteins expressed in Esherichia coli from cloned cDNAs encoding human sTnT isoforms. C: predominant MHC-I fibers in CMT1 and CMT2 single muscle fibers randomly selected from tibialis anterior muscle biopsies in the present study were confirmed by glycerol-SDS-PAGE and mAb FA2 Western blots of representative samples.