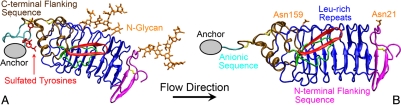
Fig. 5.
Model for flow alignment of GPIbαN. Anchored to the end of a long stalk, the N-terminal domain of GPIbα extends ∼60 nm from the platelet membrane, making it an ideal flow sensor as the platelet circulates in the blood stream. Depending on the GPIbαN-stalk anchor, flow may exert different forces on the U-shaped protein (blue) and the presence/absence of two potential N-glycans (orange) at Asn-21 and Asn-259 to align GPIbαN with the flow direction (indicated) differently, as shown in A and B, respectively. The anchor is mediated by contacts of the anionic sequence (cyan) with other portion of the C-terminal flanking sequence (ochre) through three sulfated tyrosines (red sticks), which are likely to affect the alignment of GPIbαN and, in turn, the β-switch region in the flow field. Our model hypothesizes that the alignment in A exposes the β-switch [drawn in both loop (green) and β-hairpin (red) conformations] to the flow stream, which induces the loop-hairpin transition, thereby enhancing the on-rate for GPIbα-VWF binding. Mutations that replace the three sulfated tyrosines or remove the N-glycans may alter the GPIbαN alignment with flow as shown in B, which affects the exposure of the β-switch to the flow, thereby altering the flow enhancement of GPIbα-VWF binding.