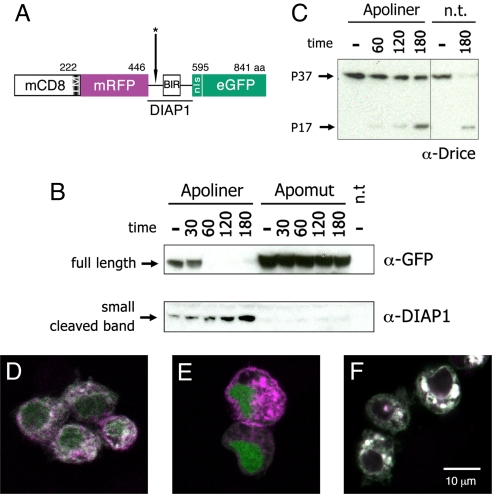
Fig. 1.
Structure and cleavage of Apoliner. (A) Schematic representation of Apoliner. Numbers indicate position of residues. The cleavage site is marked with an asterisk (see Materials and Methods). (B, C ) Western blots showing Drice, Apoliner, and Apomut at various times following induction of reaper expression in S2 cells. Numbers indicate time in minutes; and minus signs indicate uninduced cells. Apoptosis was induced 48 h after transfection of a vector expressing either Apoliner or Apomut. n.t., no transfection. (B Upper) Western blot stained with antiGFP to detect uncleaved (full length) Apoliner extracted from cells transfected with Apoliner or Apomut. This band has essentially disappeared at 60 min, indicating Apoliner cleavage. (B Lower) As full length Apoliner disappears, a cleavage product, detected with anti-DIAP1 increases. This fragment should also be detectable by anti-GFP, but this is unfortunately masked by a protein of similar size recognized nonspecifically by the anti-GFP antibody (data not shown). Note the absence of any cleavage product in extracts from cells expressing Apomut, a strong indication that Apoliner cleavage depends on the integrity of the caspase cleavage site. Note also that Drice cleavage (appearance of the P17 Drice fragment in C) only begins to be faintly detectable 60 min after induction, a time when full length Apoliner is completely cleaved (as shown in B Upper). (D–F) S2 cells transfected with Apoliner (D, E ) or Apomut (F ), and induced to die by a 10-min ultraviolet exposure. Before induction, RFP (magenta) and GFP (green) colocalize at membranes (giving a white signal in the merge) in a majority of cells (D). After induction, GFP relocalizes to the nucleus of most cells (E; see Fig. S1 for details). Even 180 min after apoptosis induction, cleavage of Apomut could not be detected as indicated by the colocalization of GFP and RFP (F ).