Abstract
We studied myosin heavy chain (MyHC) expression and fiber type distribution in laryngeal muscles in the rabbit, cat, and baboon using immunohistochemistry with highly MyHC-specific antibodies. Two types of variation in MyHC expression were found: between muscles of different function within species and within specific muscles between species. Within species, thyroarytenoid (Ta), an adductor, had faster MyHCs and fiber type profiles than the abductor, posterior cricoarytenoid (PCA), which expressed faster MyHCs than the vocal fold tensor, cricothyroid (CT). Between species, laryngeal muscles generally expressed faster MyHCs in small animals than in larger ones: extraocular (EO) MyHC was expressed in the Ta and PCA of the rabbit but not in the cat and baboon, whereas 2B MyHC was expressed in these muscles of the cat but not of the baboon. The CT expressed only MyHC isoforms and fiber types found in the limb muscles of the same species. These results are discussed in light of the hypothesis that the between-species variations in laryngeal muscle fiber types are evolutionary adaptations in response to changes in body mass and respiratory frequency. Within-species variations in fiber types ensure that protective closure of the glottis is always faster than movements regulating airflow during respiration. (J Histochem Cytochem 56:929–950, 2008)
Keywords: larynx, muscle fiber types, myosin heavy chain, immunohistochemistry, respiration, contraction, comparative physiology, scaling
The intrinsic laryngeal muscles of mammals perform a diverse repertoire of complex movements that subserve the following important functions: airway protection, respiration, and phonation. Specific laryngeal muscles have distinct functional roles. The thyroarytenoid (Ta) muscle adducts the vocal fold to close the glottis, a movement important in airway protection and respiratory control. The posterior cricoarytenoid (PCA) abducts the vocal fold to lower airway resistance during inspiration, whereas the cricothyroid (CT) tenses the vocal cord and thus controls the pitch of the voice. These functional demands are met by a complex variety of muscle fibers types (reviewed in Hoh 2005), which express different isoforms of myosin heavy chain (MyHC). MyHC is the principle determinant of muscle fiber speed and is therefore the most important marker of muscle fiber type (Bottinelli et al. 1991).
Laryngeal muscles belong to a distinct allotype, which differs from those of limb, jaw, and extraocular (EO) muscles in the subset of MyHCs they can potentially express. Mammalian limb muscles have the potential to express slow, 2A, 2X, and 2B MyHCs, forming, respectively, the following types of fibers of increasing speeds of contraction: slow, 2a, 2x, and 2b (Lucas et al. 2000; Zhong et al. 2008). Although small eutherians such as mice and rats express the full range of these MyHCs in their limb muscles, most larger animals, including cat and baboon (Lucas et al. 2000), do not express the fastest 2B MyHC. Laryngeal muscles are potentially able to express these limb MyHC isoforms (Hoh 2005), but additionally, these muscles in small animals, including rabbits (Lucas et al. 1995; Briggs and Schachat 2000) and rats (DelGaudio et al. 1995; Rhee et al. 2004), are endowed with the capacity to express the EO MyHC, an isoform that is found only in EO muscles of larger animals. This MyHC is also known as the 2L MyHC (DelGaudio et al. 1995; Briggs and Schachat 2000) and is associated with the fastest cross-bridge kinetics among the known MyHC isoforms (Li et al. 2000).
Much of the earlier work on laryngeal muscle fiber types was based on enzyme histochemistry, which cannot be relied on to resolve MyHC composition of fibers beyond fast and slow isoforms (Hoh 2005). Recent application of SDS-PAGE of MyHCs in single muscle fibers of dogs (Wu et al. 2000c) and humans (Wu et al. 2000b), and the use of immunohistochemistry (IHC) in the rat with highly specific anti-MyHC antibodies (Rhee et al. 2004), have provided reliable data on MyHC distribution in laryngeal muscle fibers in these three species. These studies have shown considerable variations in MyHC expression in laryngeal muscle fibers between species, as well as in different laryngeal muscles within species. EO MyHC is expressed in the rabbit (Lucas et al. 1995) and the rat (Rhee et al. 2004) but not in the dog (Wu et al. 2000c) or humans (Wu et al. 2000b; Li et al. 2004), whereas 2B MyHC is found in dog laryngeal muscles but not in humans. In rats, dogs, and humans, the profile of fiber types in the Ta is faster than that of the PCA, which is faster than that of the CT, reflecting differences in isometric contraction times of these muscles in rats and dogs (Hoh 2005), and suggests that similar relationships hold for the contraction times of these muscles in human.
The MyHC expression profiles of laryngeal muscles have been studied in detail in only three species. To gain further insights into the functional significance of variations in MyHC distribution in laryngeal muscles between species and between laryngeal muscles of different function within species, we immunohistochemically compare the MyHC expression profiles in muscle fibers of the Ta, PCA, and CT in the rabbit, cat, and baboon, using highly specific monoclonal antibodies (MAbs) against the full range of MyHCs expressed in laryngeal muscles. The results suggest that between-species variations in fiber types are adaptations in response to changes in body mass and respiratory frequency, whereas within-species variations ensure that protective closure of the glottis is always faster than movements regulating airflow during respiration.
Materials and Methods
Animals and Muscle Preparation
Larynges were removed from four adult female rabbits (New Zealand white, mean body mass: 2 kg), four adult cats (either sex, mean body mass: 3.5 kg), and four baboons after euthanasia with anesthetic overdose. All surgeries and animal handling were performed in accordance with the guidelines of the Animal Research Act and the NHMRC Code of Practice for the Care and Use of Animals for Scientific Purposes and were approved by the Animal Care and Ethics Committee of the University of Sydney. Three muscles of different function, the Ta, PCA, and CT, were dissected from each larynx for IHC. The excised muscles were mounted on pieces of cork with Tissue-Tek (Miles Scientific; Elkhart, IN), frozen in isopentane cooled in liquid nitrogen, and stored in liquid nitrogen until used. Serial sections of the Ta, PCA, and CT were cut at 10 μm in a cryostat at −20C. Sections from the midregions of these muscles that contain the full complement of fibers were stained immunohistochemically and used for quantification of fiber type distribution.
IHC
Indirect IHC was carried out using horseradish peroxidase (HRP)-labeled secondary antibodies as previously described (Hoh et al. 1988). The primary antibodies used include the extensively characterized MAb NOQ7-5-4-D, specific to slow MyHC (Hoh et al. 1988), and MAb SC-71 (Schiaffino et al. 1989), specific to fast 2A MyHC, as well as three MAbs raised in this laboratory, 6H1 and 10F5, specific to fast 2X and 2B MyHCs, respectively (Lucas et al. 2000), and 4A6, which is specific to EO MyHC (Lucas et al. 1995). The specificity of 6H1 (anti-2X) has been confirmed in rabbit, cat, and baboon by IHC and through Western blots (Lucas et al. 2000). The fact that 10F5 is highly specific for mammalian 2B MyHC is indicated by Western blots in many eutherian (Lucas et al. 2000) and marsupial species (Zhong et al. 2001,2008). MAb 4A6 has been shown to be broadly EO MyHC specific in mammals, including rabbits (Lucas et al. 1995), rats (Rubinstein and Hoh 2000; Rhee et al. 2004), cats (C.A. Lucas and J.F.Y. Hoh, unpublished data), and humans (Pedrosa-Domellof et al. 2000; Li et al. 2004). The secondary antibodies used were HRP-labeled goat anti-mouse IgM (Sigma; St. Louis, MO) for MAbs 6H1 and 10F5 and HRP-labeled rabbit anti-mouse immunoglobulin antibody (Dako Corp.; Carpinteria, CA) for the other MAbs.
Quantification of Muscle Fiber Type Distribution
All quantitative studies of fiber type distribution were performed on serial sections taken at the midbelly of the muscle. The percentages of fibers expressing slow, 2A, 2X, 2B, and EO MyHCs present in each muscle were calculated based on counts using photomicrographs of tissue sections stained by different anti-MyHC MAbs. Fibers were classified as hybrid if they were clearly stained by more than one anti-MyHC MAb in serial sections. The Ta can be subdivided into two distinct regions, Ta-V and Ta-X (see Results), with different fiber type compositions. These regions were separately studied. Data from Ta-V, Ta-X, PCA, and CT muscles (n=4) of each species were obtained and averaged. Fiber type compositions of different laryngeal muscles within species, and of the same muscle between species, were compared by two-tailed t-tests, and p<0.05 was taken as significant.
Results
Fiber Types in Rabbit Laryngeal Muscles
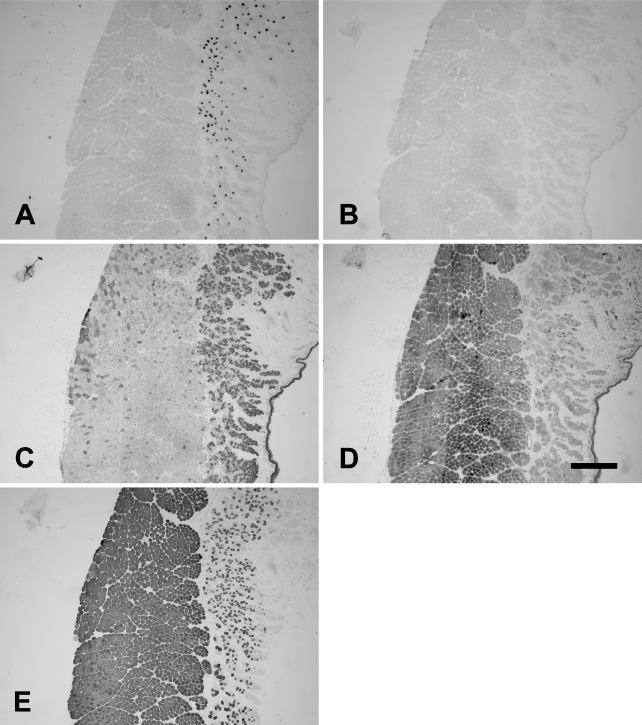
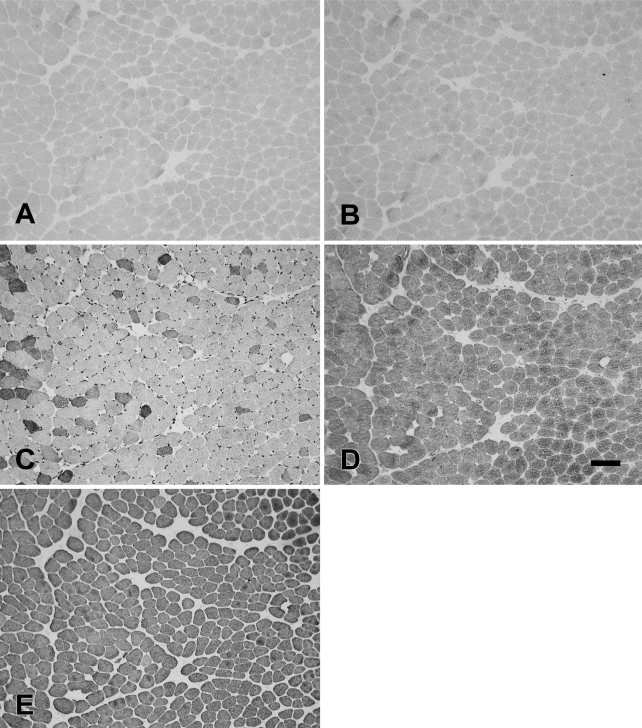
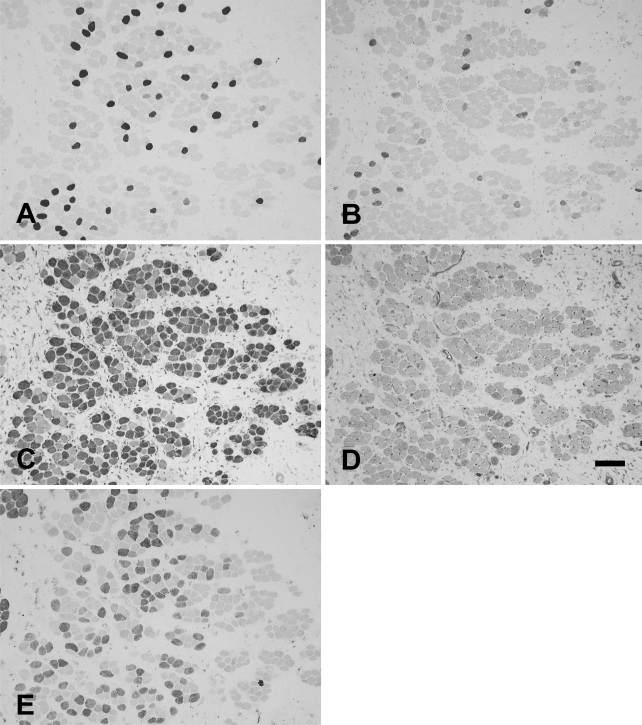
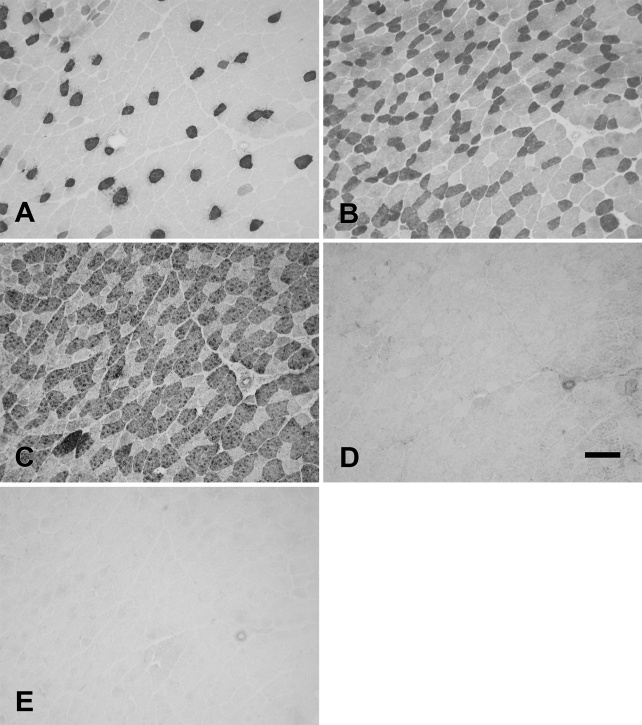
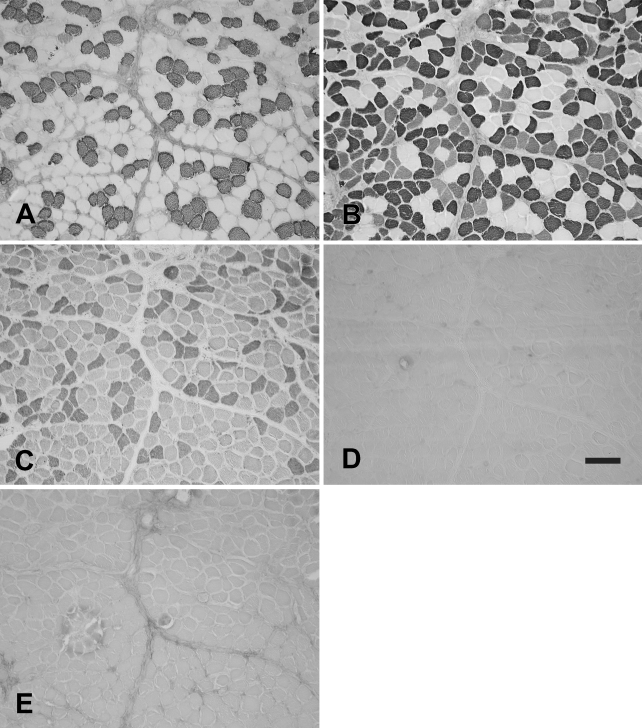
The rabbit Ta muscle was subdivided into an external and an internal (vocalis) division. The vocalis (Ta-V) division consisted of a narrow band of loosely arranged fibers lying subjacent to the vocal ligament. The major portion, the external division (Ta-X), was composed of larger, closely packed muscle fibers. Figure 1 shows the staining patterns of the MAbs to slow (Figure 1A), 2A (Figure 1B), 2X (Figure 1C), 2B (Figure 1D), and EO (Figure 1E) MyHCs. In this figure, the Ta-V is on the right, whereas the Ta-X is on the left. Under higher power (Figure 2), Ta-X is seen to be almost devoid of slow (Figure 2A) and 2a (Figure 2B) fibers. All fibers of the Ta-X expressed 2B and EO MyHCs (Figures 2D and 2E, respectively), designated as 2b/eo fibers, but among them were scattered fibers additionally coexpressing 2X MyHCs, i.e., 2x/2b/eo fibers (Figure 2C). Rabbit Ta-V (Figure 3) was made up of mostly 2x (Figure 3C) and eo fibers (Figure 3E) and scattered slow (Figure 3A), 2a (Figure 3B), and 2b (Figure 3D) fibers.
Figure 1.
Low-power photomicrographs of immunoperoxidase-stained semiserial sections of the rabbit thyroarytenoid (Ta) muscle using anti-slow monoclonal antibody (MAb) (A), anti-2A MAb (B), anti-2X MAb (C), anti-2B MAb (D), and anti-EO MAb (E). In each panel, the external division (Ta-X) is on the left and forms the bulk of the muscle, whereas the vocalis division (Ta-V) is on the right. Bar = 500 μm.
Figure 2.
Higher-power photomicrographs of immunoperoxidase-stained semiserial sections of the external division of the rabbit thyroarytenoid (Ta-X) muscle using anti-slow MAb (A), anti-2A MAb (B), anti-2X MAb (C), anti-2B MAb (D), and anti-EO MAb (E). Bar = 100 μm.
Figure 3.
Higher-power photomicrographs of immunoperoxidase-stained semiserial sections of the vocalis division of the rabbit thyroarytenoid (Ta-V) muscle using anti-slow MAb (A), anti-2A MAb (B), anti-2X MAb (C), anti-2B MAb (D), and anti-EO MAb (E). Bar = 100 μm.
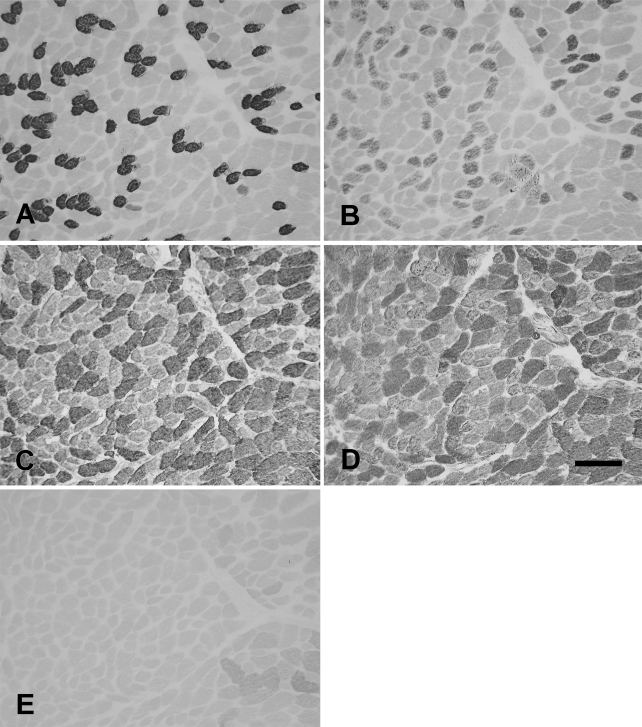
The rabbit PCA (Figure 4) was made up of slow (Figure 4A), 2a (Figure 4B), 2x (Figure 4C), and 2x/2b fibers (Figure 4D) but was devoid of eo fibers (Figure 4E).
Figure 4.
Photomicrographs of immunoperoxidase-stained semiserial sections of the rabbit posterior cricoarytenoid (PCA) muscle using anti-slow MAb (A), anti-2A MAb (B), anti-2X MAb (C), anti-2B MAb (D), and anti-EO MAb (E). Bar = 100 μm.
The rabbit CT (Figure 5) was rich in 2x (Figure 5C) and 2a fibers (Figure 5B), and to a lesser extent, slow fibers (Figure 5A). Hybrid fibers were present, expressing two or more MyHCs, but they contributed only a small percentage of total fibers.
Figure 5.
Photomicrographs of immunoperoxidase-stained semiserial sections of the rabbit cricothyroid (CT) muscle using anti-slow MAb (A), anti-2A MAb (B), anti-2X MAb (C), anti-2B MAb (D), and anti-EO MAb (E). Bar = 100 μm.
The percentages of various fiber types in the rabbit Ta-X, Ta-V, PCA, and CT derived from the four rabbits studied are presented in Table 1. As in the rat, the rabbit Ta-X consisted almost exclusively of 2b/eo fibers and clearly had the fastest fiber type profile. Although Ta-V has 19% pure eo fibers, the muscle as a whole is slowed by the presence of 61.8% of 2x fibers. The fiber type profile of PCA is slower than that of Ta-V, because PCA has no eo, 2b/eo, and 2x/2b/eo fibers, which are present in Ta-V and amounting to 27.5% of the total. However, PCA may be considered faster than CT because of the presence of 18.5% 2x/2b fibers in the PCA that are absent in the CT. It should be noted, however, that the PCA has significantly more slow fibers and less 2a and 2x fibers than the CT.
Table 1.
Distribution of laryngeal muscle fiber types in the rabbit
| Fiber type | Ta-X | Ta-V | PCA | CT |
|---|---|---|---|---|
| Slow | 0.6 ± 0.4f | 7.4 ± 2.7a | 36.0 ± 1.7e | 23.9 ± 3.1 |
| Slow/2a | 0 | 0 | 0.1 ± 0.1e | 0.9 ± 0.2 |
| Slow/2x | 0 | 0.5 ± 0.5 | 0.1 ± 0.1 | 0 |
| 2a | 0 | 1.6 ± 0.7b | 21.6 ± 2.6f | 34.2 ± 4.4 |
| 2a/2x | 0 | 0 | 0.7 ± 0.2 | 0.5 ± 0.5 |
| 2x | 0a | 61.8 ± 4.1b | 23.1 ± 3.2d | 40.5 ± 1.3 |
| 2x/2b | 0 | 1.0 ± 0.6c | 18.5 ± 2.9c | 0 |
| 2b | 0 | 0.2 ± 0.2 | 0 | 0 |
| 2x/2b/eo | 9.9 ± 2.1 | 5.2 ± 0.8c | 0 | 0 |
| 2b/eo | 89.5 ± 2.2a | 3.3 ± 0.4b | 0 | 0 |
| eo | 0b | 19.0 ± 2.7b | 0 | 0 |
Footnotes indicate statistical significance (ap<0.0001; bp<0.0005; cp<0.001; dp<0.0025; ep<0.02; fp<0.05; t-test) compared with the value on the right.
Distribution of laryngeal muscle fiber types in the rabbit. The muscles are thyroarytenoid, external division (Ta-X), thyroarytenoid, vocalis division (Ta-V), posterior cricoarytenoid (PCA), and cricothyroid (CT) muscles. The fiber types are arranged in the order of increasing speed. Values are mean percentages ± SEM; n=4 for all muscles.
Fiber Types in Cat Laryngeal Muscles
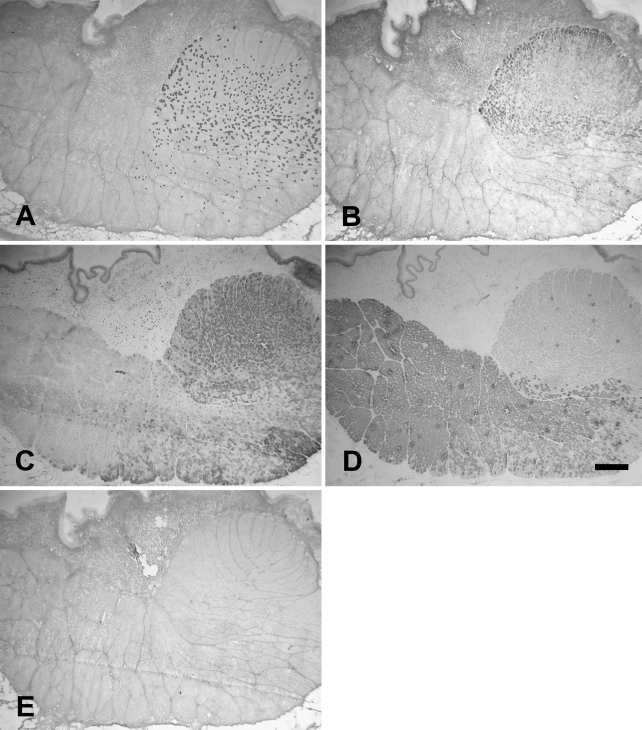
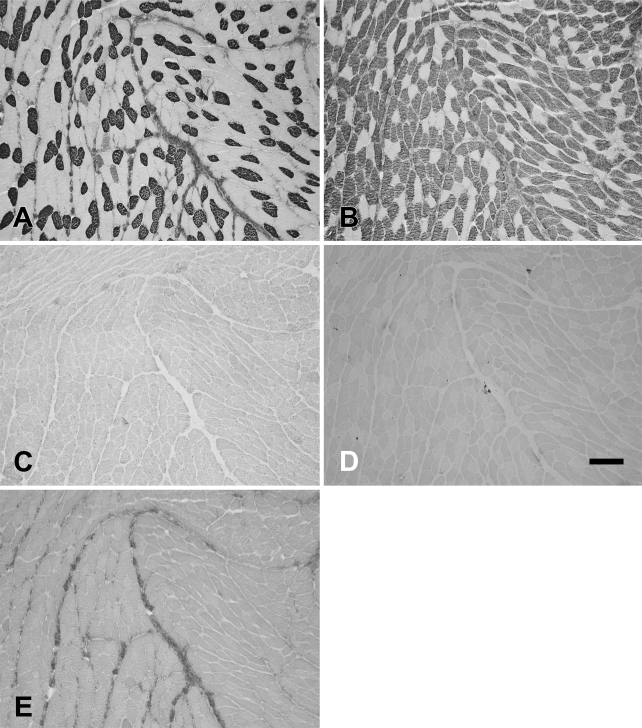
Cat Ta muscle also consisted of an external (Ta-X) and a vocalis division (Ta-V). Contrary to the rat and rabbit, however, the vocalis formed approximately one third of the whole Ta muscle mass, and their fibers were more densely packed. Figure 6 shows the staining patterns obtained with MAbs to slow (Figure 6A), 2A (Figure 6B), 2X (Figure 6C), 2B (Figure 6D), and EO (Figure 6E) MyHCs. The rounded bundle on the top right in these photomicrographs was the Ta-V, which could be clearly distinguished from the Ta-X by its contrasting fiber type composition. Under higher power (Figure 7), Ta-X could be seen to have mainly 2b (Figure 7D) and 2x fibers (Figure 7C,), with slow and 2a (Figures 7A and 7B, respectively) forming a small percentage of total fibers. In contrast, Ta-V under higher power (Figure 8) could be seen to comprise mainly 2x (Figure 8C), 2a (Figure 8B), and slow (Figure 8A) fibers. The Ta-V was devoid of 2b fibers (Figure 8D), and the cat Ta as a whole was completely devoid of eo fibers (Figure 6E).
Figure 6.
Low-power photomicrographs of immunoperoxidase-stained semiserial sections of the cat thyroarytenoid (Ta) muscle using anti-slow MAb (A), anti-2A MAb (B), anti-2X MAb (C), anti-2B MAb (D), and anti-EO MAb (E). The external division (Ta-X) forms the bulk of the muscle occupying the lower part of each panel, and the vocalis division (Ta-V) forms the upper part of each panel. Bar = 500 μm.
Figure 7.
Higher-power photomicrographs of immunoperoxidase-stained semiserial sections of the external division of the cat thyroarytenoid (Ta-X) muscle using anti-slow MAb (A), anti-2A MAb (B), anti-2X MAb (C), anti-2B MAb (D), and anti-EO MAb (E). Bar = 100 μm.
Figure 8.
Higher-power photomicrographs of immunoperoxidase-stained semiserial sections of the vocalis division of the cat thyroarytenoid (Ta-V) muscle using anti-slow MAb (A), anti-2A MAb (B), anti-2X MAb (C), anti-2B MAb (D), and anti-EO MAb (E). Bar = 100 μm.
The cat PCA muscle (Figure 9) was almost completely devoid of 2b and eo fibers, being made up principally of slow (Figure 9A), 2a (Figure 9B), and 2a/2x fibers (Figure 9C). There were some pure 2x fibers and traces of 2b/2x fibers.
Figure 9.
Photomicrographs of immunoperoxidase-stained semiserial sections of the cat PCA muscle using anti-slow MAb (A), anti-2A MAb (B), anti-2X MAb (C), anti-2B MAb (D), and anti-EO MAb (E). Bar = 100 μm.
The cat CT muscle (Figure 10) was rich in slow (Figure 10A) and 2a (Figure 10B). Scattered hybrid slow/2a fibers were present.
Figure 10.
Photomicrographs of immunoperoxidase-stained semiserial sections of the cat CT muscle using anti-slow MAb (A), anti-2A MAb (B), anti-2X MAb (C), anti-2B MAb (D), and anti-EO MAb (E). Bar = 100 μm.
The percentages of various types of fibers in the cat Ta-X, Ta-V, PCA, and CT derived from the four animals studied are presented in Table 2. Among these muscles, Ta-X has the fastest fiber type profile, which is dominated by the 78% of the fast 2b fibers not found in the other muscles. Ta-V is faster than PCA, having 51.9% of 2x fibers compared with <4% in the latter. The CT has a slower fiber type profile than the PCA, because it has no fibers faster than 2a fibers, whereas the PCA has the faster 2x/2b, 2x, and 2a/2x fibers, amounting to 24.8%.
Table 2.
Distribution of laryngeal muscle fiber types in the cat
| Fiber type | Ta-X | Ta-V | PCA | CT |
|---|---|---|---|---|
| Slow | 4.8 ± 0.9b | 19.0 ± 1.5c | 29.8 ± 1.8c | 38.7 ± 0.7 |
| Slow/2a | 0 | 0.3 ± 0.1 | 0.7 ± 0.3 | 0.8 ± 0.2 |
| 2a | 2.3 ± 0.6a | 27.1 ± 2.3d | 44.7 ± 3.4c | 60.5 ± 0.9 |
| 2a/2x | 0 | 1.5 ± 0.5 | 20.7 ± 3.6c | 0 |
| 2x | 14.5 ± 2.2a | 51.9 ± 3.0a | 3.7 ± 1.8 | 0 |
| 2x/2b | 0.4 ± 0.2 | 0.3 ± 0.1 | 0.4 ± 0.3 | 0 |
| 2b | 78.0 ± 3.1a | 0 | 0 | 0 |
Footnotes indicate statistical significance (ap<0.0001; bp<0.0002; cp<0.005; dp<0.01; t-test) compared with the value on the right.
Distribution of laryngeal muscle fiber types in the cat. The muscles are thyroarytenoid, external division (Ta-X), thyroarytenoid, vocalis division (Ta-V), posterior cricoarytenoid (PCA), and cricothyroid (CT) muscles. The fiber types are arranged in the order of increasing speed. Note that eo fibers are absent. Values are mean percentages ± SEM; n=4 for all muscles.
Fiber Types in Baboon Laryngeal Muscles
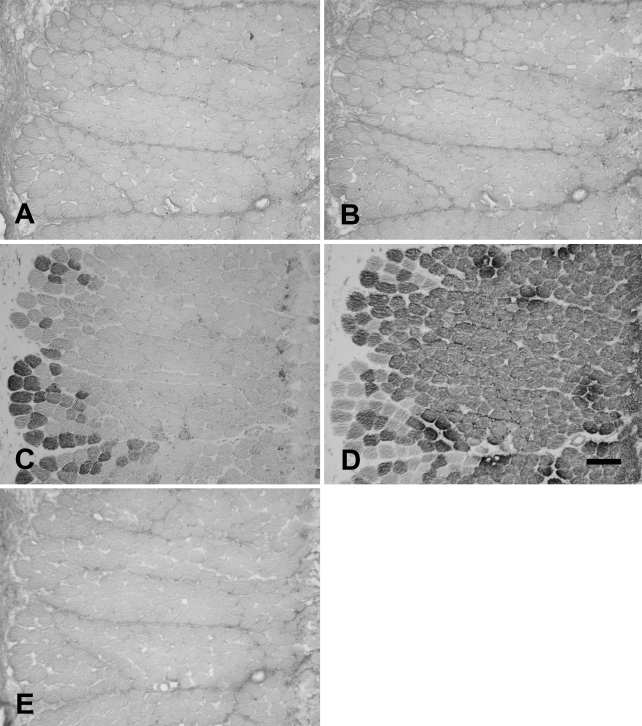
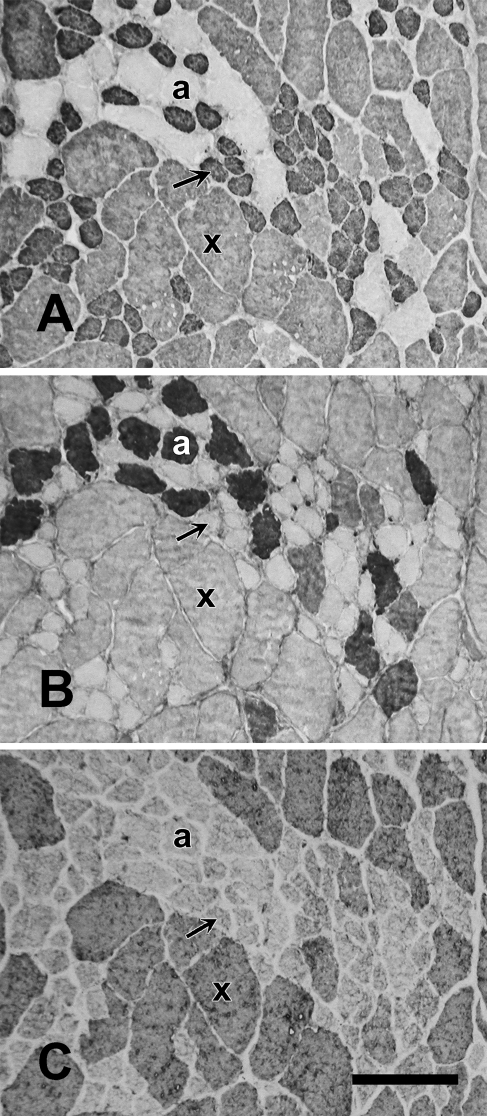
The baboon Ta muscle also consisted of distinct Ta-X and Ta-V regions that could be easily dissected out separately. The anti-slow MAb appeared to weakly cross-react with baboon 2X MyHC, as shown in Figure 11. This figure shows a high-power image of baboon Ta-V stained with anti-slow MAb (Figure 11A), anti-2A MAb (Figure 11B), and anti-2X MAb (Figure 11C). The anti-slow MAb was found to strongly stain a population of small fibers and faintly stained all fibers stained by anti-2X MAb, which were considerably larger in size, while having a very low background with fibers stained by anti-2A MAb. The consistent staining of all 2x fibers by anti-slow MAb was interpreted as a weak cross-reaction between the anti-slow MAb and 2X MyHC rather than coexpression of slow and 2X MyHCs in these fibers.
Figure 11.
High-power photomicrograph of immunoperoxidase-stained serial sections of the baboon Ta-V muscle reacting to anti-slow MAb (A), anti-2A MAb (B), and anti-2X MAb (C). Slow fibers (arrow), 2a fibers (a), and 2X fibers (x) are labeled. The anti-2A and anti-2X MAbs are specific, but the anti-slow MAb can be seen to faintly stain 2X fibers that are visibly distinct in fiber size. This cross-reaction was accounted for in quantitative analysis. Bar = 100 mm.
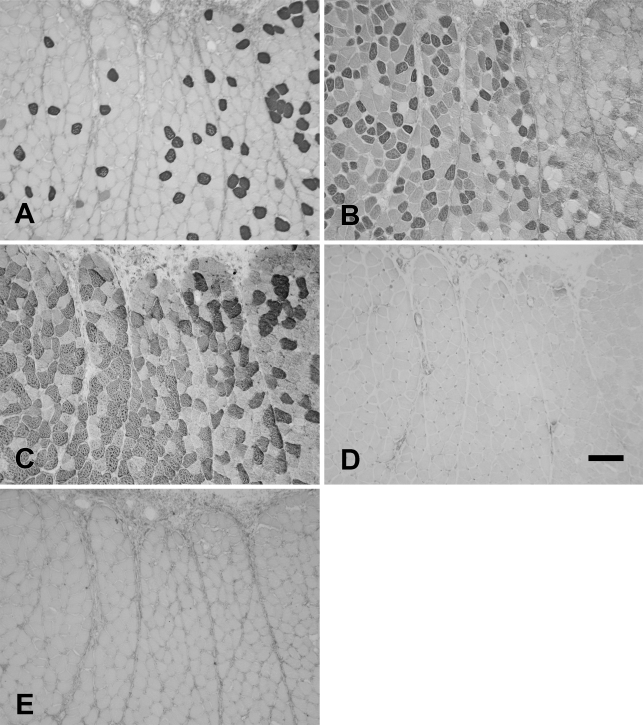
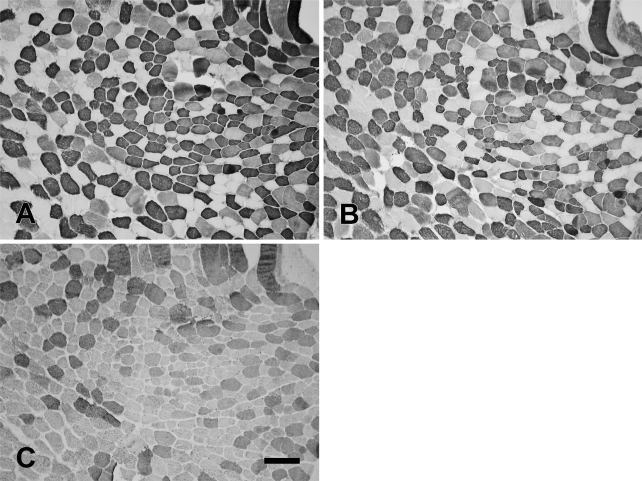
Figure 12 shows the staining pattern of the baboon Ta-X using MAbs to slow (Figure 12A), 2A (Figure 12B), 2X (Figure 12C), 2B (Figure 12D), and EO (Figure 12E) MyHCs. The Ta-X comprised predominantly pure 2a (Figure 12B; 45.9%), 2x (Figure 12C, 30.0%), and slow fibers (Figure 12A; 18.9%). Traces of slow/2a and 2a/2x hybrid fibers were also present. The baboon Ta-V and Ta-X compartments did not stain with MAbs to 2B and EO MyHCs, suggesting that these isoforms were not expressed. The fiber type composition of the baboon Ta-V (Figure 13) consisted of mostly slow (Figure 13A), 2a fibers (Figure 13B), and 2x fibers (Figure 13C).
Figure 12.
Higher-power photomicrographs of immunoperoxidase-stained semiserial sections of the external division of the baboon thyroarytenoid (Ta-X) muscle using anti-slow MAb (A), anti-2A MAb (B), anti-2X MAb (C), anti-2B MAb (D), and anti-EO MAb (E). Bar = 100 μm.
Figure 13.
Higher-power photomicrographs of immunoperoxidase-stained semiserial sections of the vocalis division of the baboon thyroarytenoid (Ta-V) muscle using anti-slow MAb (A), anti-2A MAb (B), anti-2X MAb (C), anti-2B MAb (D), and anti-EO MAb (E). Bar = 100 μm.
The baboon PCA muscle (Figure 14) was also completely devoid of 2b and eo fibers and was made up of mostly pure slow (Figure 14A), 2a (Figure 14B), and 2x fibers (Figure 14C). The fiber type profile of the baboon PCA was similar to that of the baboon Ta muscle.
Figure 14.
Photomicrographs of immunoperoxidase-stained semiserial sections of the baboon PCA muscle using anti-slow MAb (A), anti-2A MAb (B), and anti-2X MAb (C). Bar = 100 μm.
The baboon CT was composed of mostly 2a (Figure 15B) and slow fibers (Figure 15A), with a significant proportion of slow/2a hybrid fibers. It was devoid of 2x, 2b, and eo fibers (Figures 15C–15E, respectively).
Figure 15.
Photomicrographs of immunoperoxidase-stained semiserial sections of the baboon CT muscle using anti-slow MAb (A), anti-2A MAb (B), anti-2X MAb (C), anti-2B MAb (D), and anti-EO MAb (E). Bar = 100 μm.
The percentages of various fiber types in the baboon Ta-X, Ta-V, PCA, and CT derived from the four animals studied are presented in Table 3. Ta-X has a faster fiber type profile compared with Ta-V, because the former had significantly more 2x and 2a fibers and significantly less slow fibers compared with the latter. Ta-X also has a faster fiber type profile than PCA: although its higher contents of 2x and 2a fibers are not significantly different compared with those of the PCA, its slow fiber content is significantly lower (p<0.005, t-test). In this species, the Ta-V is slower than the PCA, because the former has significantly more slow fibers while other fiber types are not significantly different. The PCA, however, has a clearly faster fiber type profile than the CT, because the former has 2x and 2a/2x fibers amounting to 25.6% of total, and these fibers are absent in the latter.
Table 3.
Distribution of laryngeal muscle fiber types in the baboon
| Fiber type | Ta-X | Ta-V | PCA | CT |
|---|---|---|---|---|
| Slow | 18.9 ± 1.1a | 58.0 ± 2.0b | 42.5 ± 3.3 | 43.0 ± 5.8 |
| Slow/2a | 0.9 ± 0.9 | 1.5 ± 0.5 | 0.3 ± 0.4b | 11.2 ± 2.7 |
| 2a | 45.9 ± 4.3a | 20.5 ± 2.9 | 31.6 ± 4.3b | 45.8 ± 7.0 |
| 2a/2x | 4.3 ± 1.8 | 4.1 ± 0.3 | 3.3 ± 0.7a | 0 |
| 2x | 30.0 ± 2.6b | 15.9 ± 2.3 | 22.3 ± 4.4a | 0 |
Footnotes indicate statistical significance (ap<0.005; bp<0.01; t-test) compared with the value on the right.
Distribution of laryngeal muscle fiber types in the baboon. The muscles are thyroarytenoid, external division (Ta-X), thyroarytenoid, vocalis division (Ta-V), posterior cricoarytenoid (PCA), and cricothyroid (CT) muscles. The fiber types are arranged in the order of increasing speed. Note that 2b and eo fibers are absent. Values are mean percentages ± SEM; n=4 for all muscles.
Discussion
Overview of MyHC Expression of Different Laryngeal Muscles in Different Species
The availability of the full range of highly specific MAbs against all the known MyHCs expressed in laryngeal muscles enabled this comparative study on laryngeal muscles of different function in three different species to be undertaken. It should be pointed out that we could not exclude the possibility that MyHC isoforms not currently recognized as part of the laryngeal muscle repertoire might be present in some of the muscles studied. Because all fibers in these muscles were stained by at least one of the MAbs used, any unidentified novel MyHC isoform present was likely to be of relatively minor significance.
The results showed systematic variations in the pattern of MyHC isoform expression between laryngeal muscles within a given species, as well as within a given laryngeal muscle between species. Within species, the Ta, an adductor, expressed faster MyHCs and fiber type profiles than the abductor, the PCA, which expressed faster MyHCs and fiber type profiles than the vocal fold tensor, the CT. Between species, a given laryngeal muscle generally expressed faster MyHCs in small animals than in larger ones. Thus, variations between muscles within species seem to be related to their specific functional roles, whereas variations between species seem to be influenced by body mass. Furthermore, the pattern of MyHC expression in the CT, which is innervated by the superior laryngeal nerve (SLN), differs from that of the other laryngeal muscles, which are innervated by the recurrent laryngeal nerve (RLN).
Laryngeal muscle fiber types are under neural control, presumably through the different patterns of impulses they receive (Rhee et al. 2004). Although both SLN and RLN are branches of the vagus, the motoneuron pools innervating individual laryngeal muscles are distinct and are topographically arranged in the brainstem. Motoneurons for the CT are localized more anteriorly, principally in the retrofacial nucleus, whereas motoneuron pools for the other laryngeal muscles reside principally in the nucleus ambiguus (Gacek 1975; Yoshida et al. 1982). These differences, together with variations in their morphology and connectivity, provide the anatomic basis for the differential control of these muscles and for generating the different patterns of efferent neural activity required for inducing the expression of the various MyHCs in laryngeal muscle fiber types.
MyHC Expression in the Thyroarytenoid and Its Functional Significance
The two divisions of the Ta are believed to have distinct functions, and they are characterized by contrasting MyHC isoform profiles in all species studied here. The external division of the Ta (Ta-X) functions in adducting the vocal folds and is thus involved in reflex laryngeal closure (Fink and Demarest 1978). A comparison of fiber types in the Ta-X between rabbits, cats, baboons, and including published data for the rat (Rhee et al. 2004) is shown in Table 4. The high speed of contraction for the Ta relative to other laryngeal muscles in the same species may have a survival value to the animal by preventing foreign bodies from entering the lungs during inspiration. To be effective, the adductor needs to be faster relative to the abductor, which is active during inspiration. Thus, it is not surprising that the Ta-X was found to express the fastest fiber type profile among the laryngeal muscles in all species studied here, as well as in the rat (Rhee et al. 2004) and in humans (Li et al. 2004). This may represent an evolutionary adaptation to ensure that protective closure of the glottis is always faster than movements regulating airflow during respiration.
Table 4.
Distribution of fiber types in Ta-X muscles of the rat, rabbit, cat, and baboon
| Fiber type | Rat | Rabbit | Cat | Baboon |
|---|---|---|---|---|
| Slow | 0 | 0.6 ± 0.4d | 4.8 ± 0.9a | 18.9 ± 1.1 |
| Slow/2a | 0 | 0 | 0 | 0.9 ± 0.9 |
| 2a | 0 | 0d | 2.3 ± 0.6a | 45.9 ± 4.3 |
| 2a/2x | 0 | 0 | 0 | 4.3 ± 1.8 |
| 2x | 0 | 0b | 14.5 ± 2.2c | 30.0 ± 2.6 |
| 2x/2b | 0 | 0 | 0.4 ± 0.2 | 0 |
| 2b | 0 | 0a | 78.0 ± 3.10a | 0 |
| 2x/2b/eo | 0c | 9.9 ± 2.1c | 0 | 0 |
| 2b/eo | 100 ± 0.0c | 89.5 ± 2.2a | 0 | 0 |
Footnotes indicate statistical significance (ap<0.0001; bp<0.001; cp<0.005; dp<0.01, t-test) compared with the value on the right.
Distribution of fiber types in Ta-X muscles of the rat, rabbit, cat, and baboon. Data for the rat are from (Rhee et al. 2004) and are included for comparison. The fiber types are arranged in the order of increasing speed. Note that pure eo fibers are absent. Values are mean percentages ± SEM; n=4 for all species.
The Ta-X also plays a role in respiratory control by reducing the size of the glottis during expiration, thereby adjusting the resistance to airflow. Even during normal breathing, rhythmic electromyograms have been recorded in the cat Ta (Green and Neil 1955). Table 4 shows that there is a progressive shift of fiber types toward slower ones as the body size of the species increases. The significance of this will be discussed in relation to respiratory function.
The vocalis division of the thyroarytenoid (Ta-V) is subjacent to the vocal ligament, and its contraction increases the tension of the non-ligamentous portion of the vocal fold (Fink and Demarest 1978) and thus may be important in modulating sound quality during phonation. Our results (Tables 1–3) show that, in all three species, the fiber type profile of the Ta-V is slower than that of the Ta-X. Furthermore, in contrast to the Ta-X, the Ta-V is endowed with a wide variety of fiber types, potentially enabling the muscle to contract at a wide range of speeds, this range being the greatest in the rabbit. In this respect, it is similar to the rat Ta-V (Rhee et al. 2004). Such versatility of MyHC expression may enable the vocalis to modulate the mechanical properties of the vocal fold, and thus the vocal frequency range, over a wide range during phonation. The fact that rats are capable of generating a high- and low-frequency ultrasonic vocalization with distinct emotional significance (Sadananda et al. 2008) may depend on the wide range Ta-V muscle fiber speeds. It is of interest that rabbit Ta-V contained 19% of pure eo fibers, whereas rat Ta-V contained <3% (Rhee et al. 2004). This may have functional significance for vocalization in the rabbit. In view of the neural regulation of laryngeal fiber types, the presence of such a high proportion of pure eo fibers shows that EO MyHC is not always coregulated with 2B MyHC in 2b/eo fibers but that a distinct signal, presumably an impulse pattern, exists to cater for the exclusive expression of the EO MyHC.
MyHC Expression in the PCA and Its Functional Significance
The PCA functions as the only abductor of the vocal fold, and its primary role is controlling the size of the glottis during respiration. The vocal folds are rhythmically abducted by the PCA during inspiration and adducted by the other intrinsic laryngeal muscles during expiration (Green and Neil 1955). EMG studies showed that PCA muscle activity started before phrenic nerve activity and inspiratory airflow (Berkowitz et al. 1997). In the rat, the contraction time of the PCA is slightly longer than that of the very fast Ta muscle (Hinrichsen and Dulhunty 1982). The fiber type profiles of PCA muscles in this study support the notion that the PCA is intermediate in speed between the Ta and the CT in each species (Tables 1–3). Table 5 collates the fiber type distributions of the PCA of the three species studied here and includes the published data in the rat (Rhee et al. 2004). It can be seen that there is a trend for the fiber type profile to slow down in the direction of increasing body size: rat → rabbit → cat. However, the profile of fiber types of the baboon is, arguably, comparable to that of the cat.
Table 5.
Distribution of fiber types in PCA muscles of the rat, rabbit, cat, and baboon
| Fiber type | Rat | Rabbit | Cat | Baboon |
|---|---|---|---|---|
| Slow | 7.4 ± 1.4a | 36.0 ± 1.7g | 29.8 ± 1.8f | 42.5 ± 3.3 |
| Slow/2a | 0 | 0.1 ± 0.1 | 0.7 ± 0.3 | 0.3 ± 0.4 |
| 2a | 16.6 ± 2.2 | 21.6 ± 2.6c | 44.7 ± 3.4 | 31.6 ± 4.3 |
| 2a/2x | 0.6 ± 0.2 | 0.7 ± 0.2c | 20.7 ± 3.6d | 3.3 ± 0.7 |
| 2x | 29.1 ± 1.7 | 23.1 ± 3.2c | 3.7 ± 1.8e | 22.3 ± 4.4 |
| 2x/2b | 0b | 18.5 ± 2.9b | 0.4 ± 0.3 | 0 |
| 2b | 44.6 ± 1.6a | 0 | 0 | 0 |
| 2x/2b/eo | 0 | 0 | 0 | 0 |
| 2b/eo | 1.9 ± 1.0 | 0 | 0 | 0 |
Footnotes indicate statistical significance (ap<0.0001; bp<0.001; cp<0.002; dp<0.005; ep<0.01; fp<0.02; gp<0.05; t-test) compared with the value on the right.
Distribution of fiber types in PCA muscles of the rat, rabbit, cat, and baboon. Data for the rat are from (Rhee et al. 2004) and are included for comparison. The fiber types are arranged in the order of increasing speed. Note that pure eo fibers are absent. Values are mean percentages ± SEM; n=4 for all species.
MyHC Expression in the CT and Its Functional Significance
Previous works have shown that the CT in the rat (Wu et al. 2000a), dog (Wu et al. 2000c), and human (Wu et al. 2000b) may only express isoforms of MyHC found in their limb muscles. Our findings in rabbit, cat, and baboon are in general agreement: EO MyHC, absent in rabbit limb muscle, is absent in rabbit CT, and 2B MyHC, which is absent in cat limb muscle (Lucas et al. 2000), is also absent in cat CT as well. Functionally consistent with this general observation, the CT in several species has a similar isometric contraction time to that of fast limb muscle in the same species (Martensson and Skoglund 1964; Hall-Craggs 1968). These results suggest that the CT, innervated by the SLN, may differ allotypically from the other laryngeal muscles innervated by the RLN. These muscles have distinct developmental origins; the CT is derived from the fourth branchial arch, whereas the RLN-innervated muscles are from the sixth arch (Sperber 1989). However, as pointed out above, baboon (this study) and human (Li et al. 2004) Ta and PCA muscles do not express EO or 2B MyHC, making them allotypically indistinguishable from the CT and limb muscles using MyHC expression as a criterion. An alternative hypothesis for the fact that the CT does not express EO and/or 2B isoforms in these species is simply that it is the slowest of the laryngeal muscles and consequently has the slowest MyHC profile relative to other laryngeal muscles and thus excludes these fast isoforms. Whether the CT is allotypically different from other laryngeal muscles may be resolved by attempting to induce EO or 2B expression by cross-innervating the CT with the RLN.
Fiber type distributions of the CT in the rat, rabbit, cat, and baboon are listed in Table 6 for comparison. As with the Ta and PCA, an increase in body mass is reflected in a slowing of fiber type profile in the CT, with 2b fibers present only in the rat, 2x fibers being abundant in the rat and rabbit but absent in cat and baboon, and the proportion of slow fibers being smallest in the rat and highest in the baboon. This again reflects the respiratory role of this muscle. In quiet breathing, the CT is active predominantly in inspiration but increases its inspiratory and expiratory activity when respiration is stimulated by hypercapnia (Mathew et al. 1988).
Table 6.
Distribution of fiber types in CT muscles of the rat, rabbit, cat, and baboon
| Fiber type | Rat | Rabbit | Cat | Baboon |
|---|---|---|---|---|
| Slow | 19.0 ± 1.5 | 23.9 ± 3.1c | 38.7 ± 0.7 | 43.0 ± 5.8 |
| Slow/2a | 0 | 0.9 ± 0.2 | 0.8 ± 0.2e | 11.2 ± 2.7 |
| 2a | 12.5 ± 2.1c | 34.2 ± 4.4d | 60.5 ± 0.9 | 45.8 ± 7.0 |
| 2a/2x | 0.3 ± 0.3 | 0.5 ± 0.5 | 0 | 0 |
| 2x | 61.2 ± 3.2b | 40.5 ± 1.3a | 0 | 0 |
| 2x/2b | 2.1 ± 1.0 | 0 | 0 | 0 |
| 2b | 4.9 ± 0.8b | 0 | 0 | 0 |
Footnotes indicate statistical significance (ap<0.0001; bp<0.001, cp<0.005; dp<0.002, ep<0.01; t-test) compared with the value on the right.
Distribution of fiber types in CT muscles of the rat, rabbit, cat, and baboon. Data for the rat are from (Rhee et al. 2004) and are included for comparison. The fiber types are arranged in the order of increasing speed. Note that eo and 2b fibers are absent in rabbit, cat, and baboon CT. Values are mean percentages ± SEM; n=4 for all species.
Variations in MyHC Expression in Limb/Trunk Muscles Across Species
Before considering variations in MyHC expression in laryngeal muscles of various mammalian species, it is instructive to consider such variations in limb/trunk muscles. These muscles consume the bulk of metabolic energy in an animal, and kinetic properties of their MyHCs would be expected to be fine tuned by the locomotory demands on these muscles during evolution. In adult limb muscles of the smaller species such as mouse, rat, rabbit, and guinea pig, all three fast MyHC isoforms (2A, 2X, 2B) are expressed (Bar and Pette 1988; Gorza 1990; Schiaffino and Reggiani 1994). For small animals to survive in the wild, they need to compensate for their small size by evolving intrinsically fast but energetically expensive locomotory muscle fibers to avoid predation. In larger animals, long limbs reduce the need for fast intrinsic muscle speed, and these animals can conserve energy by reducing the speeds of their muscle fibers. Body size influences the contractile properties of a muscle through two distinct mechanisms: changing the ATPase activities of myosin isoforms and changing the fiber type profile of a given muscle.
Changes in ATPase activities of homologous myosins in response to body mass result in changes in Vo, the unloaded speed of shortening, of fibers containing them. Data from rats, rabbits, humans, and horses, spanning a 1200-fold change of body mass, showed that Vo of slow muscle fibers scaled to (body mass)−0.18 (Rome et al. 1990), whereas the allometric coefficient was −0.175 for 2a fibers, −0.098 for 2x fibers, and −0.048 for 2b fibers (Pellegrino et al. 2003).
Changes in fiber type profile in a given muscle as a function of body mass can be seen in slow and fast muscles at the low and high ends of the mass spectrum, respectively. The slow soleus muscles in cats (Hoh et al. 1988), rabbits (Hoh and Yeoh 1979), and guinea pigs (Asmussen and Marechal 1989) are composed of virtually pure slow fibers. In smaller animals such as rats and mice, the soleus muscle has a large population (∼30%) of 2a fibers in addition to slow fibers (Asmussen and Marechal 1989). In very small mammals such as shrews, there are very few slow fibers in any limb muscle; the majority of their locomotory system is composed of fast fibers (Savolainen and Vornanen 1995), the soleus being made up of 87% 2x fibers. In fast muscles of most large animals, such as humans (Smerdu et al. 1994), carnivores (Talmadge et al. 1996), ruminants (Tanabe et al. 1998), and horses (Rivero et al. 1999), the fastest 2b fibers are permanently absent. However, recent studies showed that dropping out of 2b fibers is not a universal mechanism for adapting to large body mass, because they are found in adult pigs (Lefaucheur et al. 1998), llamas (Graziotti et al. 2001), and kangaroos (Zhong et al. 2001).
Variations in MyHC Expression in Laryngeal Muscles Across Species
The results of this study show that, between species, a given laryngeal muscle generally expressed faster MyHCs in small animals than in larger ones, just as in limb muscles. However, to understand why the Ta and PCA of rats, rabbits, and cats express isoforms not found in their respective limb muscles, we need to consider their role in respiratory control. In all mammals, the vocal folds act as regulatory valves to the flow of air in and out of the lungs. As pointed out above, the vocal folds are rhythmically abducted by the PCA during inspiration and adducted by the other intrinsic laryngeal muscles during expiration. A fundamental requirement for the respiratory system in any animal is to meet the needs of its energy metabolism. The following discussion shows that the variations in fiber types in laryngeal muscles between species is intimately linked to the control of airway resistance and respiratory frequency in relation to the metabolic rate of the animal, which is linked to the body mass.
A consideration of the physiology of scaling gives insights into the correlations between MyHCs expressed in the laryngeal muscles, body mass, and metabolic rate. Basal metabolic rate of eutherian and marsupial mammals scale approximately to (body mass)0.75 (Kleiber 1961; Dawson and Hulbert 1970; Schmidt-Nielson 1984). The specific metabolic rate, i.e., metabolic rate per unit body mass, consequently scales to (body mass)−0.25. This means that the metabolic rate of a given mass of tissue from a small animal is higher than that from a larger animal, and the supply of oxygen, and consequently, the ventilation of the lungs, needs to increase proportionally. Because lung volumes do not change as a fraction of body size (Tenney and Remmers 1963), the respiratory system can cope with changes in specific metabolic rate as a result of changes in body mass solely by varying the frequency of breathing. Thus, with respect to body mass, the respiratory frequency has to scale to the same exponent as metabolic rate, i.e., −0.25. In close agreement, empirical data showed that respiratory frequency scales to (body mass)−0.26 (Schmidt-Nielson 1984). This means that small animals must have higher respiration rates than their larger counterparts and that laryngeal muscles of small animals have less time within which to regulate airflow during each breath. Laryngeal muscles must increase in speed as body mass decreases, in conformity with the allometric equation relating respiratory frequency to body mass. As pointed out above, for isoforms expressed in limb muscles, the exponent of the allometric equation relating speed of contraction to body mass is in the range −0.048 (2b fibers) to −0.18 (slow fibers), falling short of the –0.26 needed to meet requirements for respiratory control; this problem is particularly acute for 2b fibers. This means that, as body mass decreases, the increase in speed of a given fiber type, achieved by changing myosin ATPase activity of each isoform while apparently appropriate to meet locomotory demands, is not fast enough for respiratory control. A shift in fiber type distribution toward faster fibers would thus be necessary.
Our findings presented in Tables 4–6 amply confirm the expectation that, as body mass decreases, a shift in fiber types in laryngeal muscles toward faster fibers occurs. In going from baboon to cat, this takes the form of expressing the fastest limb isoform, 2B MyHC, which is not expressed in cat limb muscles. As body mass decreased further in rabbits and rats, 2b fibers are not fast enough for laryngeal functions, thus necessitating the recruitment of the fastest known MyHC isoform, EO, which is not found in limb muscles of any species.
Cats are not very much larger than rabbits and yet have completely lost EO MyHC expression, whereas rabbits have not. This suggests that phylogenetic relationship may also play a role in determining the difference in repertoire of MyHC expression in laryngeal muscles between species. This role, if present, is likely to be small, in view of the strength of the relationship between body mass and respiratory frequency. A more extensive study is needed to assess this possibility. Our hypothesis that between-species variations in fiber types reflect adaptations in response to changes in body mass and respiratory frequency would predict that EO MyHC, and probably even 2B MyHC, will not be expressed in the Ta of the largest rodent, the capybara (body mass ∼50 kg), and 2B MyHC will be absent in the Ta of large cats such as tigers and lions.
Predominance of Hybrid Fibers in Laryngeal Muscles
A striking feature of laryngeal muscle fiber types is the predominance of fibers coexpressing two or more MyHCs. Wu and colleagues analyzed MyHC compositions of single laryngeal muscle fibers by SDS-PAGE in dogs (Wu et al. 1998,2000c), rats (Wu et al. 2000a), and humans (Wu et al. 2000b) and found that, in these species, there were substantial proportions of hybrid fibers. For example, in rat Ta, 100% of fibers, in the dog PCA, ∼20% of fibers, and in the human PCA, up to 40% of fibers were found to be hybrid fibers. IHC analysis in rat laryngeal muscles confirmed the prevalence of 2b/eo fibers (100%) in rat Ta-X (Rhee et al. 2004).
Using IHC with monospecific antibodies, this study shows that there is considerable variation in the prevalence of hybrid fibers among laryngeal muscles in the three species studied (Tables 1–3). In the rabbit Ta-X, nearly all fibers are hybrids, as in the rat (Rhee et al. 2004). However, Ta muscles in the cat and baboon have <6% of hybrid fibers. The PCA muscles of the rabbit and cat have moderately high proportions of hybrid fibers (19–22%), although it is relatively low (3.6%) in the baboon. Thus, high hybrid fiber content is not a universal feature of laryngeal muscles. It has been suggested that the prevalence of hybrid fibers in some laryngeal muscles may be a reflection of the complex functional demands made on them and results from the convergence of various types of neural regulatory signals when the same motor units are used for different types of tasks (Hoh 2005). The reason for the variability of hybrid fiber abundance between muscles in the same species, and in homonymous muscles between species, may be caused by variations in their patterns of use.
Acknowledgments
This work was supported by a grant from the National Health and Medical Research Council of Australia.
References
- Asmussen G, Marechal G (1989) Maximal shortening velocities, isomyosins and fibre types in soleus muscle of mice, rats and guinea-pigs. J Physiol 416:245–254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar A, Pette D (1988) Three fast myosin heavy chains in adult rat skeletal muscle. FEBS Lett 235:153–155 [DOI] [PubMed] [Google Scholar]
- Berkowitz RG, Sun QJ, Chalmers J, Pilowsky PM (1997) Respiratory activity of the rat posterior cricoarytenoid muscle. Ann Otol Rhinol Laryngol 106:897–901 [DOI] [PubMed] [Google Scholar]
- Bottinelli R, Schiaffino S, Reggiani C (1991) Force-velocity relations and myosin heavy chain isoform compositions of skinned fibres from rat skeletal muscle. J Physiol 437:655–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briggs MM, Schachat F (2000) Early specialization of the superfast myosin in extraocular and laryngeal muscles. J Exp Biol 203:2485–2494 [DOI] [PubMed] [Google Scholar]
- Dawson TJ, Hulbert AJ (1970) Standard metabolism, body temperature, and surface areas of Australian marsupials. Am J Physiol 218:1233–1238 [DOI] [PubMed] [Google Scholar]
- DelGaudio JM, Sciote JJ, Carroll WR, Escalmado RM (1995) Atypical myosin heavy chain in rat laryngeal muscle. Ann Otol Rhinol Laryngol 104:237–245 [DOI] [PubMed] [Google Scholar]
- Fink BR, Demarest RJ (1978) Laryngeal Biomechanics. Cambridge, Harvard University Press
- Gacek RR (1975) Localization of laryngeal motor neurons in the kitten. Laryngoscope 85:1841–1861 [DOI] [PubMed] [Google Scholar]
- Gorza L (1990) Identification of a novel type 2 fiber population in mammalian skeletal muscle by combined use of histochemical myosin ATPase and anti-myosin monoclonal antibodies. J Histochem Cytochem 38:257–265 [DOI] [PubMed] [Google Scholar]
- Graziotti GH, Rios CM, Rivero JL (2001) Evidence for three fast myosin heavy chain isoforms in type II skeletal muscle fibers in the adult llama (Lama glama). J Histochem Cytochem 49:1033–1044 [DOI] [PubMed] [Google Scholar]
- Green JH, Neil E (1955) The respiratory function of the laryngeal muscles. J Physiol 129:134–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall-Craggs ECB (1968) The contraction times and enzyme activity of two rabbit laryngeal muscles. J Anat 102:241–255 [PMC free article] [PubMed] [Google Scholar]
- Hinrichsen C, Dulhunty A (1982) The contractile properties, histochemistry, ultrastructure and electrophysiology of the cricothyroid and posterior cricoarytenoid muscles in the rat. J Muscle Res Cell Motil 3:169–190 [DOI] [PubMed] [Google Scholar]
- Hoh JF (2005) Laryngeal muscle fibre types. Acta Physiol Scand 183:133–149 [DOI] [PubMed] [Google Scholar]
- Hoh JFY, Hughes S, Hale PT, Fitzsimons RB (1988) Immunocytochemical and electrophoretic analyses of changes in myosin gene expression in cat limb fast and slow muscles during postnatal development. J Muscle Res Cell Motil 9:30–47 [DOI] [PubMed] [Google Scholar]
- Hoh JFY, Yeoh GPS (1979) Rabbit skeletal myosin isoenzymes from foetal, fast-twitch and slow-twitch muscles. Nature 280:321–323 [DOI] [PubMed] [Google Scholar]
- Kleiber M (1961) The Fire of Life. An Introduction to Animal Energetics. New York, Wiley
- Lefaucheur L, Hoffman RK, Gerrard DE, Okamura CS, Rubinstein N, Kelly A (1998) Evidence for three adult fast myosin heavy chain isoforms in type II skeletal muscle fibers in pigs. J Anim Sci 76:1584–1593 [DOI] [PubMed] [Google Scholar]
- Li ZB, Lehar M, Nakagawa H, Hoh JF, Flint PW (2004) Differential expression of myosin heavy chain isoforms between abductor and adductor muscles in the human larynx. Otolaryngol Head Neck Surg 130:217–222 [DOI] [PubMed] [Google Scholar]
- Li ZB, Rossmanith GH, Hoh JF (2000) Cross-bridge kinetics of rabbit single extraocular and limb muscle fibers. Invest Ophthalmol Vis Sci 41:3770–3774 [PubMed] [Google Scholar]
- Lucas CA, Kang LH, Hoh JF (2000) Monospecific antibodies against the three mammalian fast limb myosin heavy chains. Biochem Biophys Res Commun 272:303–308 [DOI] [PubMed] [Google Scholar]
- Lucas CA, Rughani A, Hoh JFY (1995) Expression of extraocular myosin heavy chain in rabbit laryngeal muscle. J Muscle Res Cell Motil 16:368–378 [DOI] [PubMed] [Google Scholar]
- Martensson A, Skoglund CR (1964) Contractile properties of intrinsic laryngeal muscles. Acta Physiol Scand 60:318–336 [DOI] [PubMed] [Google Scholar]
- Mathew OP, Sant'Ambrogio FB, Woodson GE, Sant'Ambrogio G (1988) Respiratory activity of the cricothyroid muscle. Ann Otol Rhinol Laryngol 97:680–687 [DOI] [PubMed] [Google Scholar]
- Pedrosa-Domellof F, Holmgren Y, Lucas CA, Hoh JFY, Thornell LE (2000) Human extraocular muscles: unique pattern of myosin heavy chain expression during myotube formation. Invest Ophthalmol Vis Sci 41:1608–1616 [PubMed] [Google Scholar]
- Pellegrino MA, Canepari M, Rossi R, D'Antona G, Reggiani C, Bottinelli R (2003) Orthologous myosin isoforms and scaling of shortening velocity with body size in mouse, rat, rabbit and human muscles. J Physiol 546:677–689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhee HS, Lucas CA, Hoh JF (2004) Fiber types in rat laryngeal muscles and their transformations after denervation and reinnervation. J Histochem Cytochem 52:581–590 [DOI] [PubMed] [Google Scholar]
- Rivero JL, Serrano AL, Barrey E, Valette JP, Jouglin M (1999) Analysis of myosin heavy chains at the protein level in horse skeletal muscle. J Muscle Res Cell Motil 20:211–221 [DOI] [PubMed] [Google Scholar]
- Rome LC, Sosnicki AA, Goble DO (1990) Maximum velocity of shortening of 3 fibre types from horse soleus muscle - implications for scaling with body size. J Physiol 431:173–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein NA, Hoh JF (2000) The distribution of myosin heavy chain isoforms among rat extraocular muscle fiber types. Invest Ophthalmol Vis Sci 41:3391–3398 [PubMed] [Google Scholar]
- Sadananda M, Wohr M, Schwarting RK (2008) Playback of 22-kHz and 50-kHz ultrasonic vocalizations induces differential c-fos expression in rat brain. Neurosci Lett 435:17–23 [DOI] [PubMed] [Google Scholar]
- Savolainen J, Vornanen M (1995) Fiber types and myosin heavy chain composition in muscles of common shrew (Sorex araneus). J Exp Zool 271:27–35 [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Gorza L, Sartore S, Saggin L, Ausoni S, Vianello M, Gundersen K, et al. (1989) Three myosin heavy chain isoforms in type 2 skeletal muscle fibres. J Muscle Res Cell Motil 10:197–205 [DOI] [PubMed] [Google Scholar]
- Schiaffino S, Reggiani C (1994) Myosin isoforms in mammalian skeletal muscle. J Appl Physiol 77:493–501 [DOI] [PubMed] [Google Scholar]
- Schmidt-Nielson K (1984) Scaling: Why Animal Size is So Important. Cambridge, Cambridge University Press
- Smerdu V, Karschmizrachi I, Campione M, Leinwand L, Schiaffino S (1994) Type IIx myosin heavy chain transcripts are expressed in type IIb fibers of human skeletal muscle. Am J Physiol Cell Physiol 36:C1723–1728 [DOI] [PubMed] [Google Scholar]
- Sperber GH (1989) Craniofacial Embryology. London, Wright
- Talmadge RJ, Grossman EJ, Roy RR (1996) Myosin heavy chain composition of adult feline (Felis catus) limb and diaphragm muscles. J Exp Zool 275:413–420 [DOI] [PubMed] [Google Scholar]
- Tanabe R, Muroya S, Chikuni K (1998) Sequencing of the 2a, 2x, and slow isoforms of the bovine myosin heavy chain and the different expression among muscles. Mamm Genome 9:1056–1058 [DOI] [PubMed] [Google Scholar]
- Tenney SM, Remmers JE (1963) Comparative quantitative morphology of the mammalian lung: diffusing area. Nature 197:54–56 [DOI] [PubMed] [Google Scholar]
- Wu YZ, Baker MJ, Crumley RL, Blanks RH, Caiozzo VJ (1998) A new concept in laryngeal muscle: multiple myosin isoform types in single muscle fibers of the lateral cricoarytenoid. Otolaryngol Head Neck Surg 118:86–94 [DOI] [PubMed] [Google Scholar]
- Wu YZ, Baker MJ, Crumley RL, Caiozzo VJ (2000a) Single-fiber myosin heavy-chain isoform composition of rodent laryngeal muscle: modulation by thyroid hormone. Arch Otolaryngol Head Neck Surg 126:874–880 [DOI] [PubMed] [Google Scholar]
- Wu YZ, Crumley RL, Armstrong WB, Caiozzo VJ (2000b) New perspectives about human laryngeal muscle: single-fiber analyses and interspecies comparisons. Arch Otolaryngol Head Neck Surg 126:857–864 [DOI] [PubMed] [Google Scholar]
- Wu YZ, Crumley RL, Caiozzo VJ (2000c) Are hybrid fibers a common motif of canine laryngeal muscles? Single-fiber analyses of myosin heavy-chain isoform composition. Arch Otolaryngol Head Neck Surg 126:865–873 [DOI] [PubMed] [Google Scholar]
- Yoshida Y, Miyazaki T, Hirano M, Shin T, Kanaseki T (1982) Arrangement of motoneurons innervating the intrinsic laryngeal muscles of cats as demonstrated by horseradish peroxidase. Acta Otolaryngol 94:329–334 [DOI] [PubMed] [Google Scholar]
- Zhong WW, Lucas CA, Hoh JF (2008) Myosin isoforms and fibre types in limb muscles of Australian marsupials: adaptations to hopping and non-hopping locomotion. J Comp Physiol [B] 78:47–55 [DOI] [PubMed] [Google Scholar]
- Zhong WW, Lucas CA, Kang LH, Hoh JF (2001) Electrophoretic and immunochemical evidence showing that marsupial limb muscles express the same fast and slow myosin heavy chains as eutherians. Electrophoresis 22:1016–1020 [DOI] [PubMed] [Google Scholar]