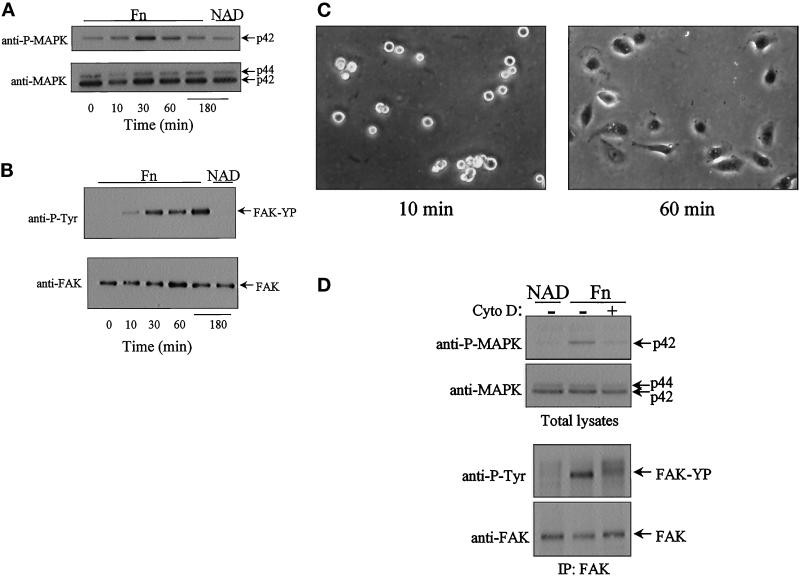
Figure 3.
Adhesion to fibronectin activates both MAPK and FAK tyrosine phosphorylation: relationship to cell spreading and cytoskeletal organization. Confluent ECV304 cells were serum starved for 6 to 8 h. Detached cells were suspended in medium containing 2% BSA. Following a 1-h period in suspension, cells were allowed to attach to dishes coated with fibronectin (Fn). For nonadherent conditions, cells were held in suspension (NAD). At the intervals indicated, cells were lysed and analyzed by Western blot analysis for MAPK activation (A) and FAK tyrosine phosphorylation (B) from FAK immunoprecipitates (top panels, A and B). Parallel blots were probed with either a polyclonal anti-MAPK or a polyclonal anti-FAK antibody to show equal loading (bottom panels, A and B). (C) Phase-contrast micrographs of cells after 10 and 60 min on fibronectin show cell attachment at the early time point and attachment and spreading at the later time point. (D) Cells were pretreated with cytochalasin D (Cyto D, 1 μM) for 20 min prior to replating on fibronectin for 30 min and analyzed for both MAPK activation (top panels) and FAK tyrosine phosphorylation (bottom panels).