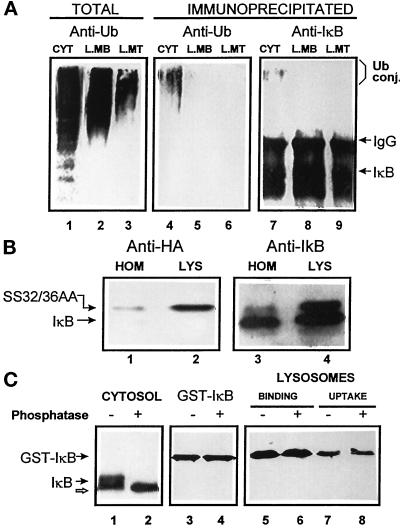
Figure 6.
Lysosome-associated IκB is not ubiquitinated or phosphorylated. (A) Cytosol (100 μg of protein), lysosomal membranes, and lysosomal matrix (derived from 100 μg of lysosomal protein) were isolated as described in MATERIALS AND METHODS and then directly subjected to SDS-PAGE (lanes 1–3) or immunoprecipitated with a specific antibody against IκB (lanes 4–9). Filters were immunoblotted with a specific antibody against ubiquitin (lanes 1–6) or against IκB (lanes 7–9). (B) Homogenate and lysosomes isolated from WEHI231 cells stably transformed with a HA epitope-tagged SS32/36AA IκB. Samples (50 μg of protein) were subjected to SDS-PAGE and immunoblot with a specific antibody against HA (lanes 1 and 2) or against IκB (lanes 3 and 4). (C) Rat liver cytosol (isolated in the presence of phosphatase inhibitors, 100 μg of protein, lanes 1 and 2) or purified GST-IκB (lanes 3–8) were treated with phosphatase as described in MATERIALS AND METHODS and then subjected to SDS-PAGE and immunoblot with a specific antibody against IκB. Binding and uptake of phosphatase-treated and untreated GST-IκB by isolated rat liver lysosomes (lanes 5–8) was analyzed as described in Figure 4A.