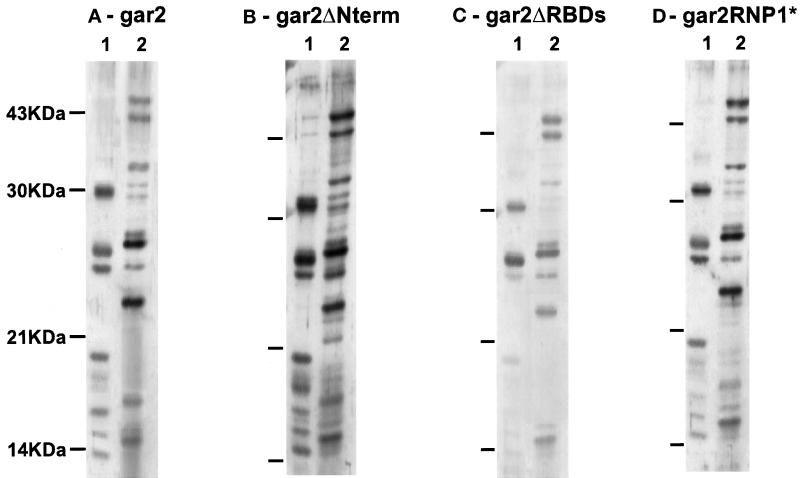
Figure 6.
Far-Western blots on S. pombe ribosomal proteins from the small (1) or the large (2) subunit. Blots were incubated with 5 μg/ml recombinant gar2–T7 (A), gar2ΔNterm–T7 (B), gar2ΔRBDs–T7 (C), or gar2RNP1*–T7 (D) proteins. Protein–protein interactions were revealed by immunostaining with anti-T7 antibodies and ECL. The full-length gar2 protein interacts in vitro with ribosomal proteins from both subunits (A), and affinity of gar2ΔNterm for ribosomal proteins is identical to that of wild-type gar2 (B). The affinity of gar2ΔRBDs for ribosomal proteins in vitro is generally low, suggesting strong alteration of gar2 structure after the deletion of the RBDs. Only a few interactions are significantly lost (C). gar2RNP1* has the same affinity for ribosomal proteins in vitro as wild-type gar2 (D).