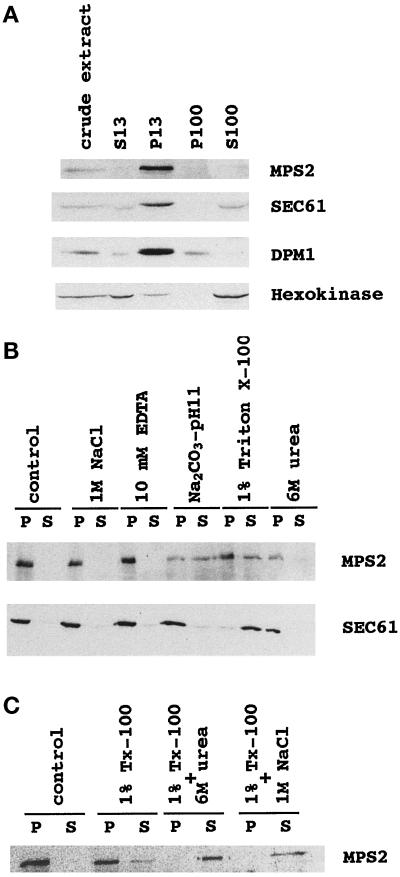
Figure 4.
9xmycN-Mps2p fractionates as an integral membrane protein. A whole-cell extract was prepared from cells expressing 9xmycN-Mps2p from its normal promoter (MCL120) by gentle spheroplast lysis (see MATERIALS AND METHODS) and then fractionated as described in MATERIALS AND METHODS. (A) Cell fractionation: the same amount of protein from the whole-cell extract and S13, P13, P100, and S100 fractions were subjected to SDS-PAGE and immunoblotted with anti-myc, anti-Sec61p, anti-Dpm1p, and anti-hexokinase antibodies successively. (B) Solubilization of Mps2p from the P13 fraction prepared from MCL120. The pellet was extracted with lysis buffer (control) or lysis buffer containing 1 M NaCl, 50 mM Tris (pH 7.5)-10 mM EDTA, 0.2 M Na2CO3 (pH 11), 1% Triton X-100, or 6 M urea as described in MATERIALS AND METHODS. The mixtures were incubated on ice for 10 min and then centrifuged to produce a pellet fraction (P) and a supernatant fraction (S). Equivalent samples of the pellet and supernatant were analyzed by immunoblotting with anti-myc or anti-Sec61 antibodies. (C) Total solubilization of Mps2p protein from the P13. The P13 pellet was extracted with lysis buffer (control) or lysis buffer containing 1% Triton X-100, 1% Triton X-100 plus 6 M urea, or 1% Triton X-100 plus 1 M NaCl. Pellet (P) and supernatant (S) were separated and analyzed by immunoblotting.