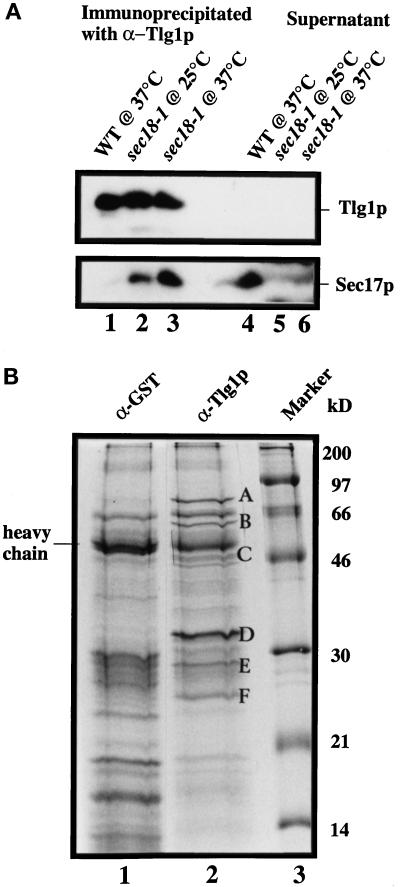
Figure 9.
Characterization of Tlg1p-containing SNARE complex. Spheroplasts of sec18-1 cells were incubated at either 25 or 37°C for 1 h and lysed with Triton X-100, and extracts were prepared as described (see MATERIALS AND METHODS). (A) Twenty milligrams of the extract from the indicated cells were incubated with anti-Tlg1p antibodies (200 μg), which had been covalently coupled to cyanogen bromide–activated Sepharose CL4B (400 μg). The supernatants and eluted immunoprecipitates were TCA precipitated, and  of the samples was analyzed by immunoblot for Tlg1p and Sec17p. Sec17p was not coimmunoprecipitated by anti-Tlg1p antibodies when extracts from wild-type cells were used (lanes 1 and 4). However, Sec17p was coimmunoprecipitated by anti-Tlg1p antibodies when sec18-1 cell extracts were used (lanes 2 and 3), particularly when spheroplasts were preincubated at 37°C (lane 3). (B) Large-scale immunoprecipitation of the Tlg1p–SNARE complex from sec18-1 cells. Proteins immunoprecipitated with anti-GST antibodies (lane 1) or anti-Tlg1p antibodies (lane 2) were resolved on a 12% SDS-PAGE gel and stained with Coomassie brilliant blue. The molecular mass markers are shown in lane 3. The heavy chain of the rabbit antibody is detectable despite the chemical cross-linking. All of the specific protein bands denoted by different letters were individually excised and sequenced for identity (refer to Table 3).
of the samples was analyzed by immunoblot for Tlg1p and Sec17p. Sec17p was not coimmunoprecipitated by anti-Tlg1p antibodies when extracts from wild-type cells were used (lanes 1 and 4). However, Sec17p was coimmunoprecipitated by anti-Tlg1p antibodies when sec18-1 cell extracts were used (lanes 2 and 3), particularly when spheroplasts were preincubated at 37°C (lane 3). (B) Large-scale immunoprecipitation of the Tlg1p–SNARE complex from sec18-1 cells. Proteins immunoprecipitated with anti-GST antibodies (lane 1) or anti-Tlg1p antibodies (lane 2) were resolved on a 12% SDS-PAGE gel and stained with Coomassie brilliant blue. The molecular mass markers are shown in lane 3. The heavy chain of the rabbit antibody is detectable despite the chemical cross-linking. All of the specific protein bands denoted by different letters were individually excised and sequenced for identity (refer to Table 3).