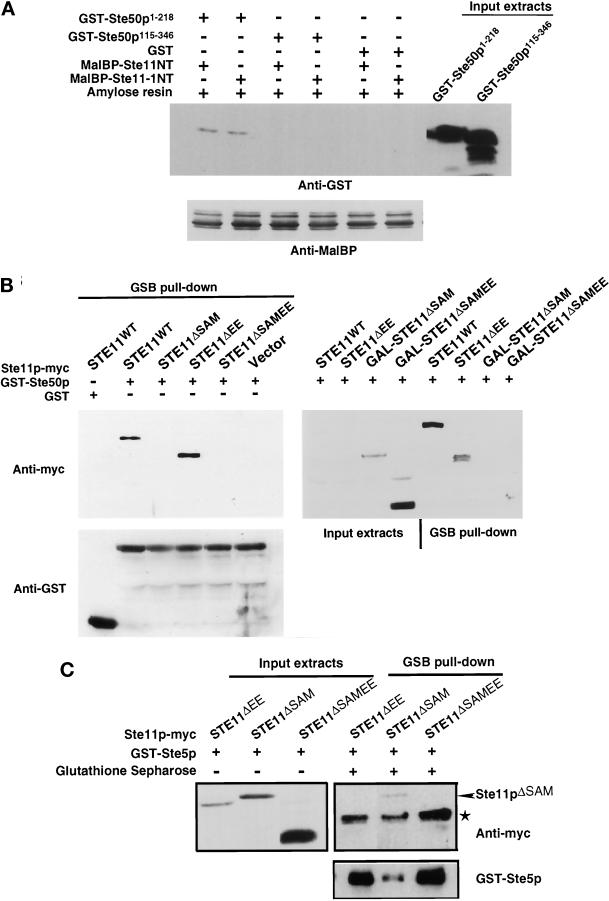
Figure 5.
Physical interaction of the N-terminal regulatory domain of Ste11p with Ste50p and Ste5p. (A) The SAM domain-containing region of Ste50p is required for the interaction with the N-terminal domain of Ste11p in vitro. Bacterial extracts containing GST alone or Ste50p fragments as GST fusions were mixed with bacterial extracts containing bacterially expressed N terminus of Ste11p or Ste11–1p as MalBP fusion proteins. Amylose agarose beads were added to the mixtures, incubated, washed extensively, and subjected to SDS-PAGE, and then immunoblot analysis was performed with anti-GST or anti-MalBP polyclonal antibodies and visualized with the ECL detection system (see MATERIALS AND METHODS). The input MalBP are shown in the bottom panel, and the input Ste50p fragments as GST fusion proteins are shown on the right of the top panel. The input GST is not shown. (B) The SAM domain-containing region of Ste11p is required for the interaction with Ste50p in vivo. Yeast strain YCW555 (MATa ssk2Δ ssk22Δ ste11Δ ste50Δ) was transformed with STE11 constructs (tagged with c-myc epitope) or the cloning vector as well as with either the GST-Ste50p expression construct (pVL57) or the GST vector. Exponentially growing cells (∼5 × 108 cells) in selective medium were shifted to selective medium or to selective medium with 3% galactose (to induce expression of GAL promoter-driven Ste11p constructs) for 3 h at 30°C. All media were supplemented with 0.5 mM CuSO4 to induce GST-Ste50 expression under the CUP1 promoter. Cells were then harvested, and cell extracts were prepared as described in MATERIALS AND METHODS. The cell extracts were either analyzed by Western blotting (input extracts) or incubated with glutathione-Sepharose beads at 4°C for 2 h, and the beads were then washed and subjected to Western blotting analyses (GSB pull-down) with either anti-myc (9E10) monoclonal antibody (to detect myc-tagged Ste11p constructs, top panels) or anti-GST polyclonal antibodies (to detect GST and GST-Ste50p, bottom panel) and visualized with the ECL detection system. Although all Ste11p constructs were not detectable by Western blotting using the crude extracts when they were expressed from their own promoter, GST-Ste50p was able to pull down specifically Ste11pWT and Ste11pΔEE. Neither Ste11pΔSAM nor Ste11pΔSAMEE were able to be pulled down by GST-Ste50p, although these proteins were overexpressed and readily detectable in the crude extracts by Western blotting. (C) The EE region of the N-terminal regulatory domain of Ste11p is required for the association of Ste5p with Ste11p. Yeast strain YCW340 (MATa ssk2Δ ssk22Δ ste11Δ) was transformed with STE11 constructs (tagged with c-myc epitope) and a GST-Ste5p expression construct (pVL23). Exponentially growing cells (∼5 × 108 cells) in selective medium with 3% raffinose were shifted to selective medium with 3% galactose (to induce expression of GAL promoter-driven Ste11p constructs) and supplemented with 0.5 mM CuSO4 (to induce GST-Ste5 expression under the CUP1 promoter) for 3 h at 30°C. The experiment was then performed as described in B. * indicates a nonspecific immunoreactivity.