FIGURE 5.
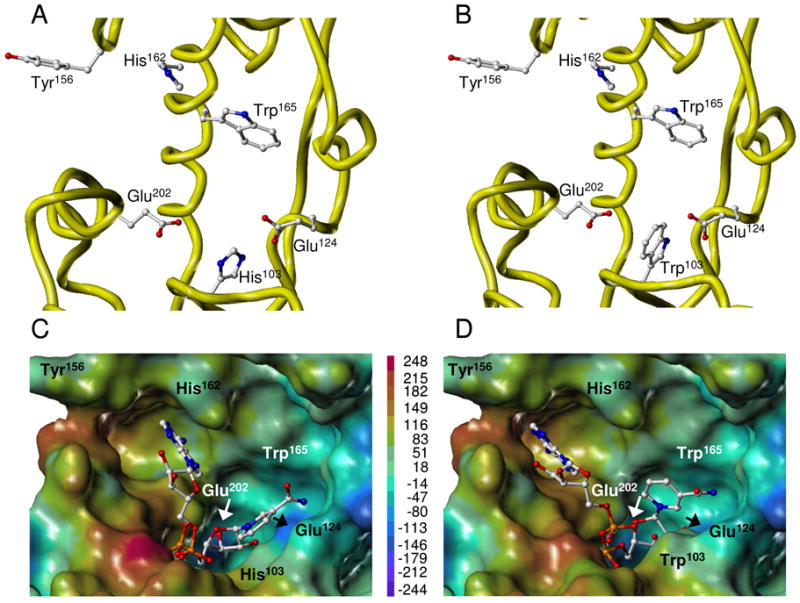
Three-dimensional structure of WT and H103W SmNACE active sites. Free WT (A) and mutant (B) enzymes. Yellow ribbons illustrate the secondary structure elements that form the catalytic cleft. Side chains of the key residues are depicted using ball-and-stick representation and colored by atom type. NAD+ docked into the active site of WT (C) and mutant (D) enzymes. The best GOLD poses (fitness values of 64 and 82, respectively) of NAD+ are shown in ball-and-stick and colored by atom type. The active site is represented as Connolly surface and colored according to the electrostatic potential computed using AMBER7 FF99 charges. The color ramp of electrostatic potential (in kcal/mol) ranges from red (most positive) to purple (most negative). The protein is always displayed in the same orientation. The rendering was performed using SYBYL ver. 7.1. Color code : white = C, red = O, dark blue = N, cyan = H and orange = P.
