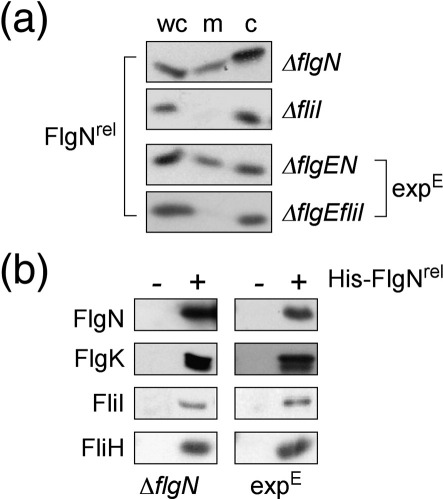
Fig. 2.
Membrane accumulation of early and late subunits in an early-locked pathway attenuated by stalling docked chaperone FlgNrel. (a) Localisation of FlgNrel (expressed in trans by 0.01% arabinose) in the whole cell (wc), membrane (m) and cytoplasm (c)11,15 of the ΔflgN and ΔfliI pathways, and in the isogenic (expE) early-locked pathways ΔflgEN and ΔflgEfliI. (b) Affinity copurification of stalled docking complexes by His–FlgNrel bait (+)11 from native ΔflgN and early-locked (expE; ΔflgEN) pathways [(−) vector-only controls]. Cell extracts were incubated with Ni–NTA resin [20 mM tris(hydroxymethyl)aminomethane–HCl pH 8.0, 300 mM NaCl and 5 mM imidazole] before washing (10 mM imidazole) and elution in SDS sample buffer. FlgN chaperone, FlgK cognate subunit and ATPase complex components FliI and FliH were detected by immunoblotting.